Nucleation kinetics of calcium oxalate monohydrate as a function of pH, magnesium, and osteopontin concentration quantified with droplet microfluidics
Kidney stone formation is a global health problem with increasing prevalence. Stone formation is a physiochemical process involving crystallization of inorganic salts in the presence of biological constituents in the urinary tract.
TU Delft researchers, F. Ibis, T. W. Yu, F. Penha, D. Ganguly, M. A. Nuhu, A. E.D.M. van der Heijden, H. J. M. Kramer and H.B. Eral have recently published a coupled in-vivo experimental and modelling study focusing on calcium oxalate monohydrate (the most common seen kidney stone type).

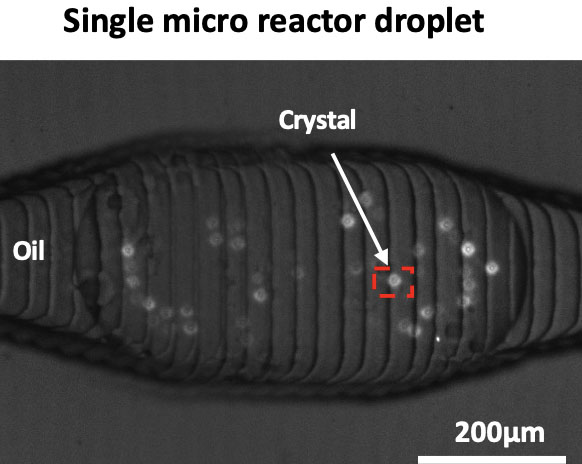
Authors quantified how fast crystals nucleate under a broad range of conditions using a droplet-based microfluidics platform. The developed platform uses of minute amounts of materials such as difficult to isolate or expensive proteins while ensuring statistically significant number of identical experiments. The study underlines the potential of microfluidics in overcoming a main challenge in kidney stone research, the overwhelming physiochemical complexity of urine.
The results are published in Biomicrofluidics (Vol.15, Issue 6).