Cell unstuck: how a glue-like protein can make our cells move
An essential aspect of the cells in our body is their ability to move, to repair certain tissues or chase intruders, for example: but how do they do it? Scientists from TU Delft, AMOLF and Utrecht University reveal how glue-like proteins called crosslinkers could not only help to hold the whole cell together passively, but surprisingly cause the cell to move as well. The research is now published in PNAS.
Up until recently, the only known mechanism to reorganize the cell’s interior was the molecular motor protein: these proteins use chemical fuel to actively transport the cell’s cytoskeleton, which controls a cell’s shape and movement. Now, this research shows that not only motors, but also crosslinkers mediate active transport. This is surprising, because crosslinkers are binding proteins that serve as a kind of glue that keeps the cell together. How these proteins could manage these seemingly contradicting actions at the same time has been unclear – up till now.
Like moving a heavy stone over tree trunks
Using purified proteins, researchers studied the interactions between two components of the cytoskeleton to better understand cell movement: actin filaments, which generate protrusive forces that drive the cell into motion, and microtubules, which control the cell’s direction. The researchers discovered that the crosslinkers connect the actin filaments and microtubules and thereby create two opposite forces: the actin will hold onto the growing tips of the microtubules, which causes the cell to be pushed forward, while at the same time the binding proteins create a backward friction. Researcher Gijsje Koenderink explains: “It turns out that the crosslinkers are able to ‘unstick’ from the actin because they are with many, and swiftly exchange places to help the actin move along the growing microtubule.”
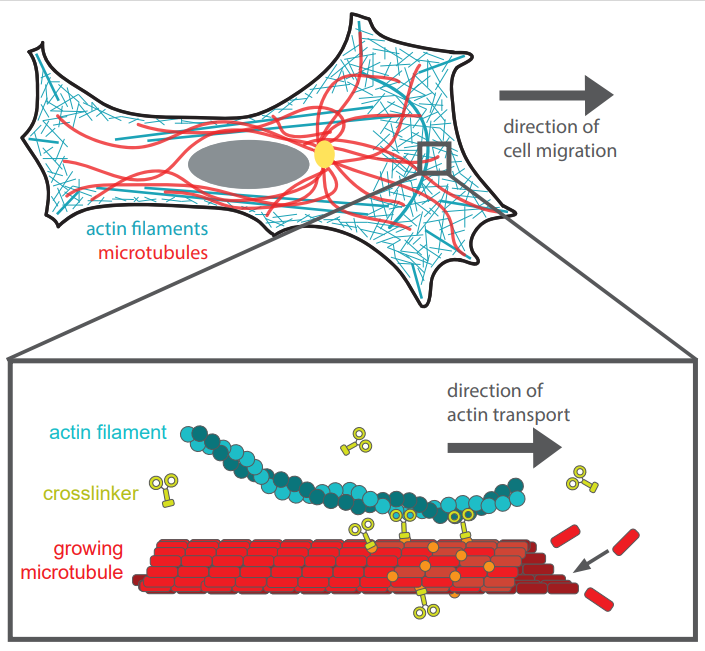
Researcher Celine Alkemade from the Koenderink and Dogterom Labs: “There are 2 movements that occur simultaneously – actin moving along crosslinkers, moving along growing microtubule. To imagine this interplay, think of transporting a heavy stone with the use of rolling tree trunks in ancient Egyptian times: here the growing microtubule are the persons who push against the tree trunks - the crosslinkers – which directly move the heavy stone – the actin filament.” Similar to how the workers need to switch or replace the rolling trunks constantly from front to back so as to transport the stone further along, so does the crosslinker unglue from the microtubules and actin, to move the cell.
New insight into how cells migrate
The way in which crosslinkers allow actin filaments to move along microtubules provides a new explanation for how cells in our body are able to migrate. Understanding how cell migration works is important to identify the molecular causes of diseases where cell migration is impaired; for instance in birth defects caused by the abnormal migration of neurons in the developing brain, as well as immunodeficiency’s which are caused by impaired immune cell migration.
On the other hand, migrating cells can cause great damage, in the case of cancer cells that metastasize from the primary tumor to other organs. Current anti-cancer drugs mainly target microtubules because of their role in cell division; these new findings point to actin-microtubule crosslinkers as a potential new drug target. Koenderink: “We now want to directly observe this mechanism in cancer cells by live-cell imaging and find out how sensitive cancer cell migration is to the presence of these crosslinkers.”
This project is part of the ERC Synergy project “MODELCELL: Building a Model Cell to Achieve Control of Cellular Organization” of prof. Marileen Dogterom and prof. Anna Akhmanova, which involved a close collaboration with prof. Gijsje Koenderink at TU Delft and prof. Pieter Rein ten Wolde at AMOLF.
