Cells: strong at the right place and time
Researchers from TU Delft and NWO institute AMOLF discovered how certain molecular bonds make living cells both flexible, in order to move, as well as strong, in order to withstand forces. Paradoxically, it turns out that these force-sensitive catch bonds are weak and inactive most of the time, but travel to specific places where and when cells become damaged. This discovery is published today in Nature Materials.
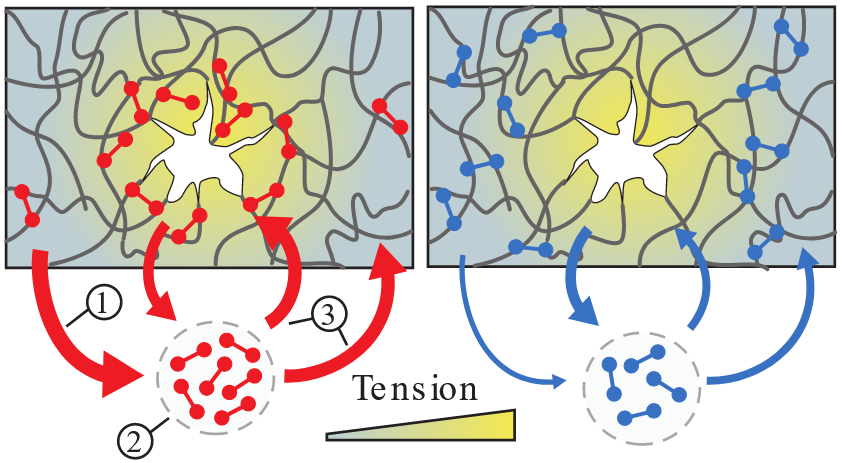
Molecular catch bond proteins can be found in many different tissues, both within and between cells. These bonds fall apart regularly, as most biological bonds do, but they have a peculiar property: if you pull hard at a catch bond, it actually starts binding tighter. Researchers discovered that this ability strengthens the material in specific places where the bond experiences stress. The discovery is a breakthrough, 20 years after the first finding of such bonds. Also, this marks the first time that the researchers have witnessed catch bonds working together within biological materials.
Both flexible and strong
Former AMOLF researcher Yuval Mulla explains: “We usually define how strong something is by one of two ways: a material can either deform well – stretch very far without breaking, such as rubber – or the material can bear much force, for instance a brick; although it’s strong, it can only stretch a little before it will break. Studying the nature of catch bonds, we found that these molecular bonds were able to do both: be flexible and strong, even though their molecular bindings are weak. And then we considered: might catch bonds explain why living cells combine the stretchiness of rubber with the strength of a brick?”
To test these ideas, the researchers measured the mechanical properties of cytoskeletal networks that they reconstituted in the lab, collaborating with the Biophysics group to pull at single bonds. They found that many of the bonds are just floating around, binding briefly only to let go again. However, when the researchers deformed the networks, they found that many bonds travel to particularly damaged sites to bind. Mulla: “Because the catch bonds accumulate at weak spots when and where they are needed to make the network very strong.”
Relation to diseases
The study included a mutant version of the same protein, one which is known to occur with a genetic disease that leads to kidney failure. Unlike a regular catch bond, the researchers found that this mutant version was always active. This increased bond strength makes it difficult for the mutant to move around, but, paradoxically, also makes the networks weaker as the bonds do not accumulate where needed, says group leader Gijsje Koenderink: “By understanding the mutant protein better, in the future we might also understand the process of kidney failure. In addition, we hope to understand how catch bonds play a role in how invasive cancer cells are.”
Material perspective on life
The research group of professor Gijsje Koenderink at Delft University of Technology is primarily interested in material properties of living matter. A central theme in her group is the fact that living cells and tissues need to be dynamic and flexible, but also strong: “This property is different from any synthetic materials we know”, says Koenderink. “Our ambition is to learn new design principles from living materials to make synthetic materials that can be both flexible and strong at the same time. In fact, we are currently working together with chemists and biophysicists like Sander Tans at AMOLF to try and make such synthetic catch bonds.”