The risk of virus inhibitors
Virus inhibitors disrupt the production of new viruses by the host cell. But too low doses can cause resistance to develop. Prof. Nynke Dekker (AS) showed how this works.
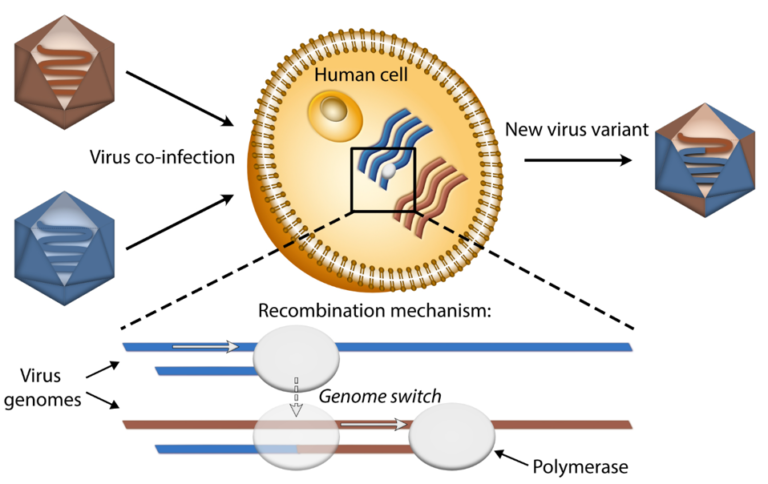
Prof. Nynke Dekker’s (Faculty of Applied Sciences) research group has unravelled the molecular origin of recombination in viruses, in other words, how new virus variants can arise. The fact that virus variants can have disastrous consequences is currently clear.
They disrupted the molecular copying machine (polymerase) that makes a copy of the RNA of a virus. Polymerase reads the building blocks of RNA chains (there are four types) and places the next building block in the right place in the copy. This is how viruses multiply. Or rather, it has itself multiplied by the genetic machinery of the infected host cell.
The sabotage comes from a molecule that closely resembles one of the building blocks, but does not fit in nicely. As a result, the copying process gets stuck and the progress of the virus is halted. At least, that is one of the possible outcomes of the antiviral drug that Dekker tested.
Other possibilities are that the copying process starts again, but makes false copies (more about this process at the end of the article). Incorrect copies will usually result in defective viruses. But by chance, a better virus may emerge that is resistant and/or more infectious than the original. This is, at least in theory, a potential risk of antiviral drugs.
Resistance
Does it happen in practice? Prof. Louis Kroes is Head of the Department of Medical Microbiology at the Leiden University Medical Centre. “We treat people with viral infections here and use antiviral drugs,” says Kroes. “This is a very different world from the molecular research that Nynke Dekker's group is doing on substances that might one day be used to fight viruses.”
He knows some examples of mutations resulting from treatment with antiviral drugs. “Lamivudine was the first agent used against Hepatitis B infection, but it was actually an anti-HIV agent. Resistance then quickly developed due to mutations in the virus.” In Kroes’ experience, starting therapy too late, using non-specific agents and not achieving complete inhibition increase the risk of resistance.
Resistance is the result of beneficial mutations. “We have now shown for the first time how an antiviral drug causes a polymerase to cause jumps in the RNA,” says Dekker enthusiastically. “As far as I know, medicines are not screened for the fact that they encourage recombination in this way.”
According to Kroes, pharmaceuticals are well aware of the risk of mutants. For example, when combating corona with Remdesivir, the manufacturer pays for the genetic analyses of the virus during treatment to keep track of mutations. As far as Kroes has seen, there was little genetic variation in the coronavirus. Is that good news? “I deduce that the virus is not under very much pressure from the drug,” he says. For this reason, the WHO advises against using the drug for Covid-19, regardless of the severity of the disease, reports Dr Richard Janissen, fellow researcher of Nynke Dekker and first author of the publication.
Magnetic tweezers
Researchers in the Nynke Dekker Lab have built a set-up with which they can closely follow a single RNA copying process. Using ‘magnetic tweezers’, they can feel exactly what happens during the genetic copying process, even when it is disrupted by a virus inhibitor. They published this in the journal Molecular Cell.
In collaboration with the University of North Carolina (USA) and Chang Gung University (Taiwan), TU Delft researchers showed that recombination can increase under pressure from viral inhibitors. Cells were infected with the virus and the amount of recombined virus RNA measured. A virus inhibitor that disrupted the RNA copying process was administered to some of the cells. Analysis showed more recombined RNA here, exactly as the molecular measurements at TU Delft had predicted. In a wider sense, this could mean that one should be wary of doses of virus inhibitors that are too low and that increase recombination but do not kill enough viruses.
Dekker emphasises that there is now more basic knowledge of these processes. “We started with molecular mechanics. Then we saw the same process in living cells. That was very interesting to experience.”