Applied Sciences’ hidden helium recovery system
Suppose you are researching quantum mechanics and you need helium to cool particles. At some point you will have to knock on Remco Roeleveld’s door. He is responsible for maintaining the helium recovery system in the Applied Sciences building.
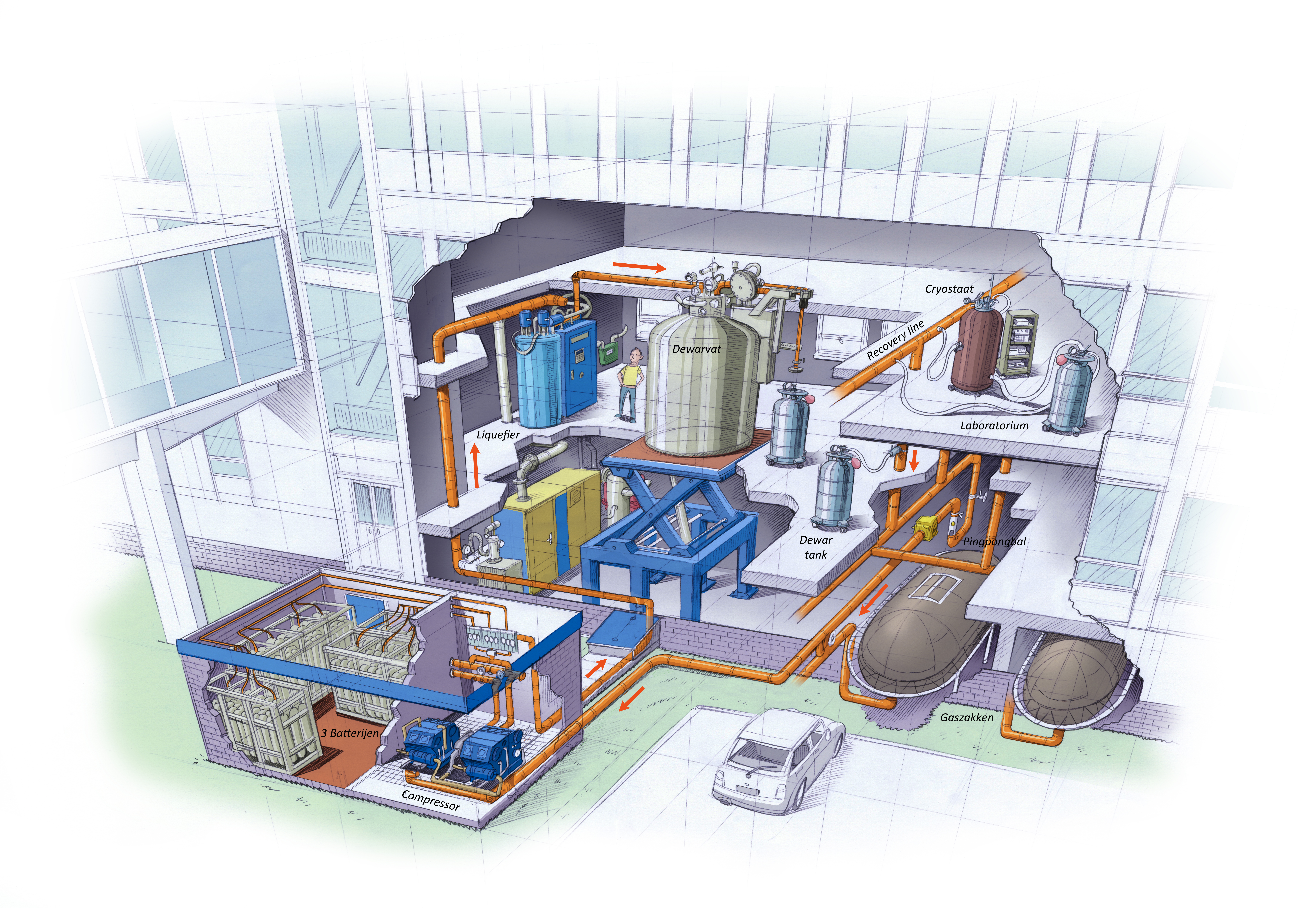
The dewar (a small tank) containing cooled, liquid helium is transported to the lab. Here, the helium is transferred to a cryostat; a device used to maintain temperatures as low as just 20 millikelvins above absolute zero. Afterwards, the helium is recycled because it is expensive stuff: “Just off the top of my head: 7.68 for one litre, not including VAT,” says Roeleveld.
The cryostat is connected to the recovery line, a pipe system that runs through the entire building. The used helium travels through these pipes to one of the four large gas storage balloons in the basement, each of which can hold 10,000 litres. Once the balloons are full, the compressor starts up and empties them. A pressure relief valve prevents them from bursting. “But we almost never have to use that,” says Roeleveld.
As soon as the gas storage balloons are full, the gas is discharged to a compressor shed. The compressor fills rows of gas cylinders (batteries) and puts them under pressure – sometimes even to 200 bar so that up to 2,760,000 litres of gaseous (i.e. 3,680 litres of liquid) helium can be stored. A computer regulates which of the batteries are to be emptied.
The helium from the batteries is returned to the building via another pipe system. Once there, the recycling can begin. The liquefier contains turbines that create a pressure difference, which cools the helium and transforms it back into a liquid. The helium then travels to the purifier, where it is cleaned. The purifier contains air and nitrogen as well as some oil, which is the result of another process. Zeolite, activated carbon particles and a low temperature are used to filter the helium. Eventually, the clean, liquid helium is transported to the large vacuum-insulated 4,300-litre dewar tank. At this stage, the helium is at a temperature of 4 kelvin and is transferred into small dewars. It is now ready to be taken back to the labs for the scientists to carry out their research.