How to make liquid electricity
It is a cruel irony that renewable energy is abundant in the summer and scarce in cold and dark winters. TU Delft researchers think they can solve the problem by converting electric energy into fuel.
TU Delft is a partner in the Power to Ammonia research programme, whereby surplus green electricity is converted to ammonia whose uses include fuel for the Nuon plant in Eemshaven.
Nuon is interested in CO2-free fuel and in seasonal electricity storage, because this could enable their gas-fired power plants in Eemshaven to play a role in a future where electricity is 100% renewable and CO2free.
The principle involves using green electricity to split water through hydrolysis, which results in hydrogen and oxygen. Hydrogen can be mixed with nitrogen extracted from the air (the atmosphere contains 78% nitrogen) and converted into ammonia (NH3) under high pressure and temperature. Unlike hydrogen gas, ammonia can be stored in liquid form in large tanks. It has a high energy density (half that of diesel) and is clean burning; if a suitable catalyst and a small amount of oxygen are used, only water vapour and nitrogen will be released.
Electrochemical Production
Professor of Energy Materials Fokko Mulder (Faculty of Applied Sciences) is searching for an alternative way to produce ammonia. Ammonia is traditionally formed using the Haber-Bosch process, which functions fine if applied at a large scale in a continuous process. “But in a sustainable future,” explains Mulder, “the electricity supply will be variable and sourced from 10 MW solar farms or wind turbines. This will require more flexible and possibly also smaller-scale ammonia production, which is why we are working on electrochemical ammonia production. One possible route involves combining hydrogen and nitrogen electrochemically into ammonia in a reactor. An alternative is to convert water and nitrogen into oxygen and ammonia. The latter would actually be more elegant, because no hydrogen is formed as an intermediate product. The Netherlands Organisation for Scientific Research NWO/TTW is currently funding the development of an electrochemical cell and the corresponding electrodes, membranes and electrolytes. Many research groups are working on this around the world, producing ammonia electrochemically remains a challenge. The response time and selectivity are proving difficult, and the pilot plants are still very small-scale.”
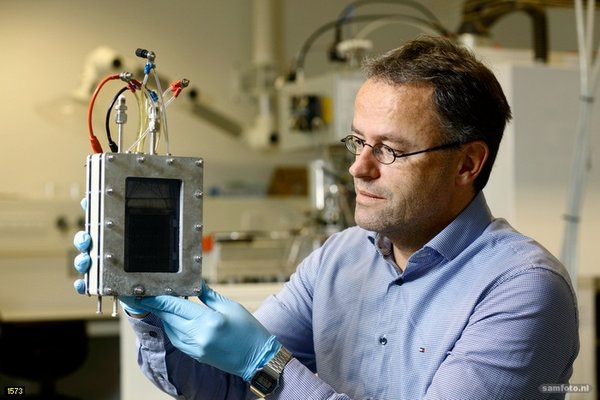
Professor Fokko Mulder shows the first version of the Battolyser, a combined nickel-iron battery and electrolyser that first charges and then produces hydrogen. Photo © Sam Rentmeester
Battolyser
Another invention conceived by Mulder’s research group, the Battolyser, could be the solution to increase hydrogen production and reduce costs. The Battolyser is a combined nickel-iron battery and electrolyser that first charges and then produces hydrogen. This means that, in principle, the device is suitable for both short-term and seasonal storage of electricity. The battery function improves the business model by helping to cover the total cost of electricity storage and so reduce the cost price of green hydrogen. Volatile and flammable hydrogen gas is the enemy of battery
makers, but in electrolysis it is actually the intention to produce as much as possible. Mulder’s research group studied the efficiency of water splitting in a nickel-iron battery and found it to be more than 80%. To date however, hydrogen production has always been seen as a disadvantage rather than an opportunity.
A number of PhD students working on various projects hope to present their results in 2021.