Our Approach
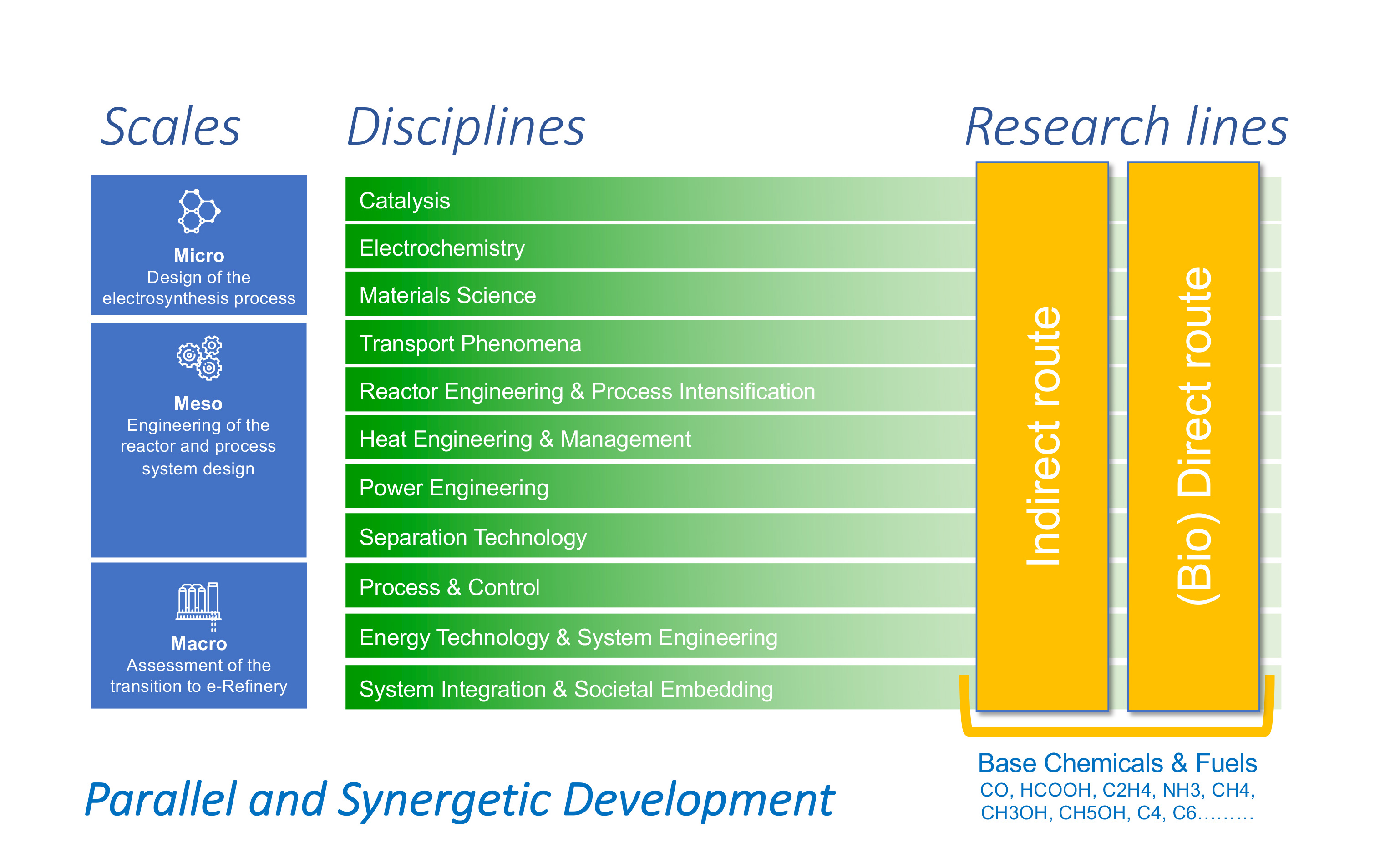
The e-Refinery program at TU Delft develops electrochemical conversion technology for the sustainable production of chemicals and fuels. For a successful application and integration of this technology in industry, we need to understand the complex interactions across multiple disciplines, time scales (nanosecond-hours) and length scales (nanometer-meter). To achieve this goal, our research addresses the fundamental questions related to performance, design and scaling.
The design of the electrosynthesis system requires in-depth knowledge of the processes involved, from the atomic to the macro scale and across the various time scales. At the Micro Scale, various aspects of the system, consisting of electrodes, electrolytes and membranes are studied by combining disciplines such as Catalysis, Material Science and Electrochemistry. At the Meso Scale, Transport Phenomena, Reactor Engineering & Process Intensification, Heat & Power Engineering and Separation Technology are needed to translate the insights obtained on the microscopic processes into practical tools to design, engineer and optimize the reactor components and process system design. At the Macro Scale, we focus on Process-, Energy- and System-Integration and Societal Embedding of the electrosynthesis systems. A timely assessment of the relevant system constraints- including the use of current assets, matching of supply and demand, evaluation of the environmental impacts, logistics and feedstock availability- will in the end determine which e-Refinery technology to use for a specific product-feed combination.
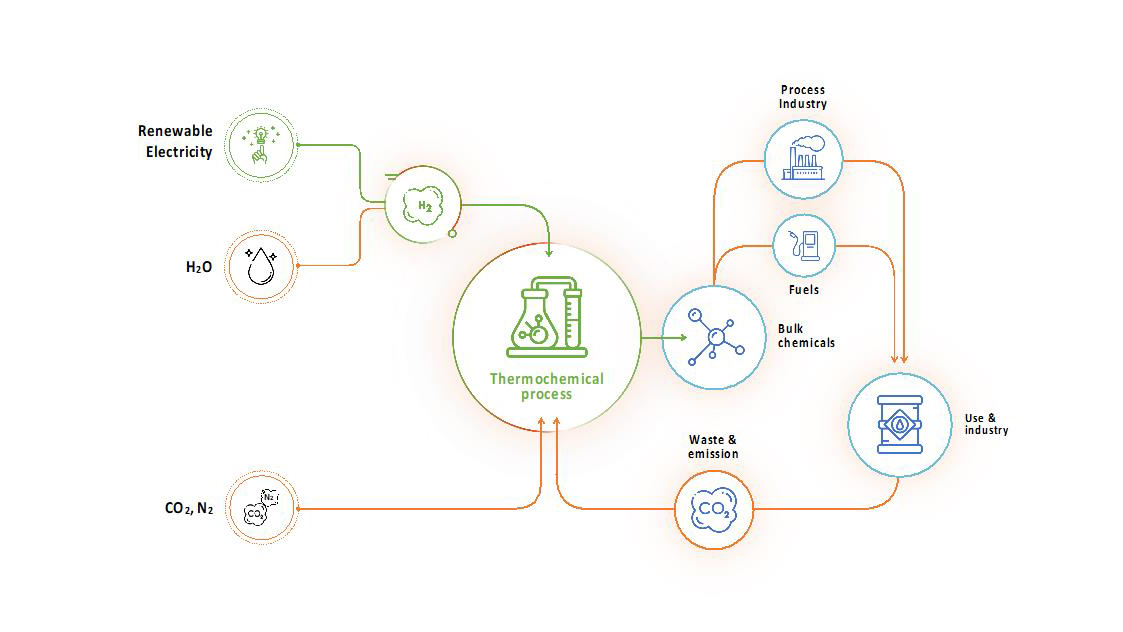
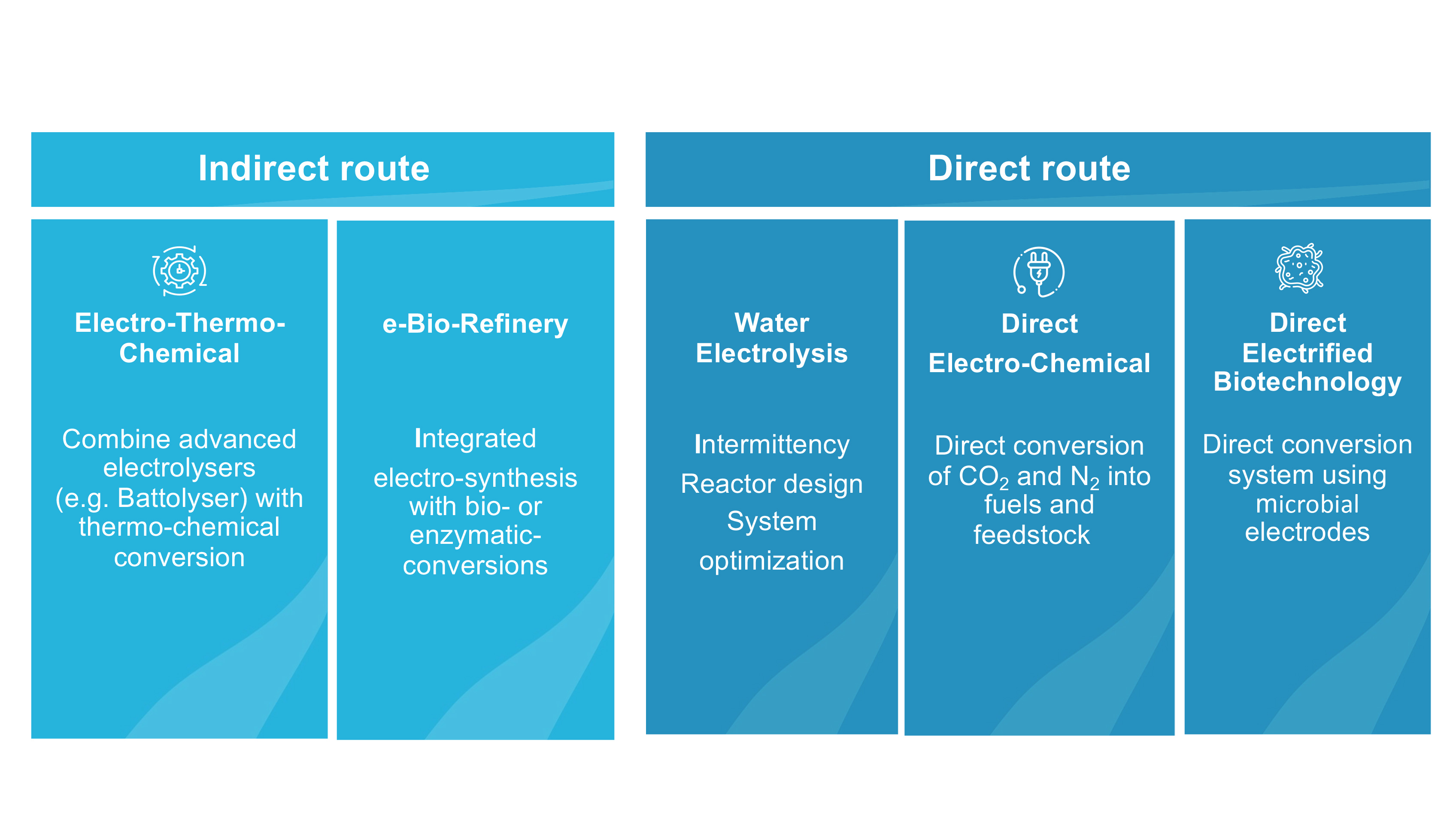
The indirect electro-thermochemical route
The indirect route produces hydrogen from water electrolysis using renewable electricity. This hydrogen can be used together with CO2 from the air, biomass or point sources or nitrogen from the air in conventional thermochemical processes (e.g., Sabatier, reverse Water Gas Shift, Fischer-Tropsch) to produce sustainable fuels or chemicals at industrial scales.
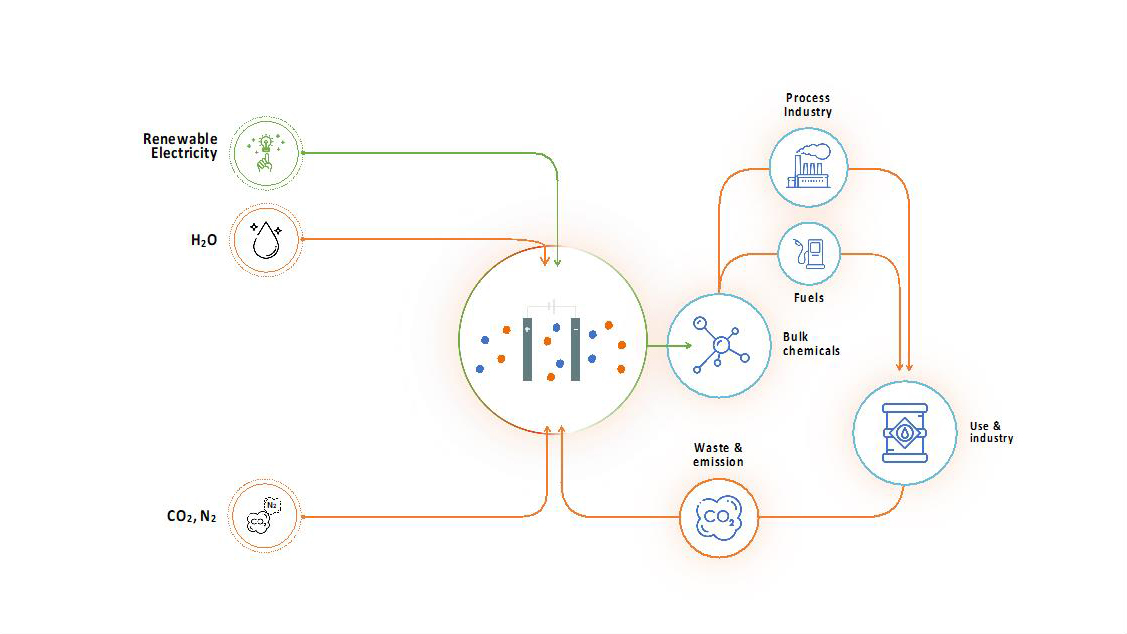
The direct electrochemical route
In the direct route, we reduce CO2 directly to base chemicals such as methanol, ethylene or CO on the catalytically active electrode surface in a CO2 electrolyser by means of renewable electricity. In addition, in the direct bio-electrochemical route a microbial electrosynthesis system (MES) is to be developed, which uses micro-organisms as catalyst to reduce CO2 to biofuels such as hexanoic acid.
Our Goal
Our goal is to demonstrate electrochemical conversion technologies within the indirect and direct route to produce base chemicals and fuels at industrial conditions and corresponding scales. In the indirect route, we have set the goal to develop a 100-kW thermal bench scale set-up of a Sabatier reactor for continuous methane production from CO2 and H2. In the direct route, design and construction of a 100-kW electrochemical testing device for the selective conversion of CO2 into ethylene is the ultimate goal.
To achieve these goals, we aim to collaborate with (inter)national academic, industrial and governmental partners to support the development and upscaling of these technologies.