Research
Probing protein oligomerization, self-assembly and aggregation
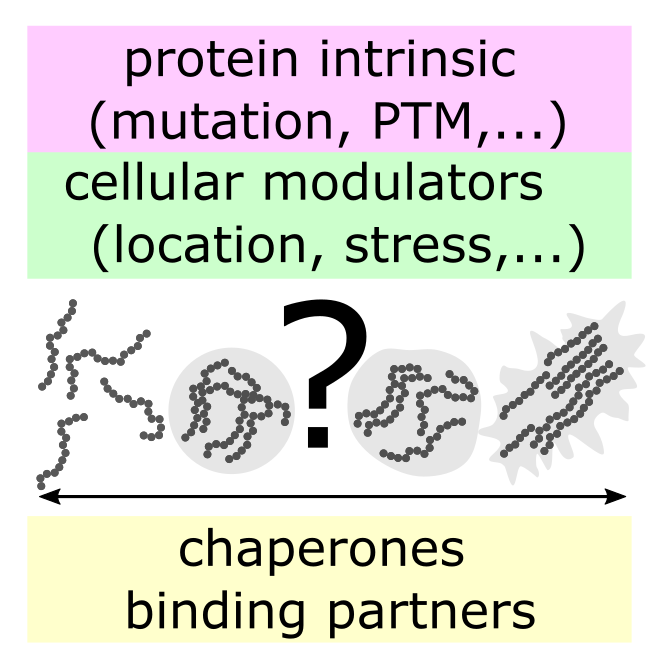
Many diseases are associated with the formation of aberrant protein aggregates, yet controlled oligomerization provides advantages for biological structure and function. Among them are neurodegenerative diseases like Alzheimer’s or Huntington’s that are to date incurable and pose one of the grand challenges for society. Despite tremendous progress it remains unclear what causes neuronal toxicity. Different types of assemblies (oligomers, fibrils, amyloid-like aggregates, …) have been identified, but the underlying molecular mechanisms of formation remain elusive. However, accumulating evidence suggests that cellular toxicity precedes the formation of insoluble inclusion bodies/plaques. The emerging paradigm of intracellular phase transitions provides a new framework for studying misregulated protein self-assembly. Still, direct evidence of these transitions in patient brains is missing. What role, if any, does liquid phase separation play in neurodegeneration? What molecular and cellular determinants influence these transitions? To answer these questions, a better definition of the structural and functional features of protein assemblies is necessary, including their mechanical properties. We will develop and use advanced (super-resolution) imaging along with other nanotechnology available in the department to tackle this challenge.
Multiplane microscopy
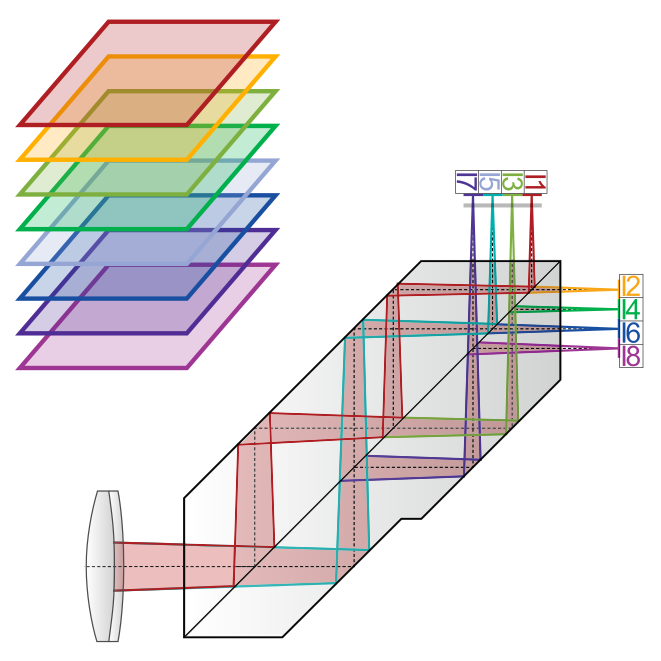
Cells are three-dimensional objects and intracellular motion is generally not constrained to a single plane. Multiplane microscopy allows visualization of cellular structures and dynamics through the simultaneous imaging of different focal planes within the sample. We realize multi-plane microscopy using image splitting prisms for ultrafast (ms), inter-plane drift-free 3D data acquisition. This implementation allows imaging across the visible spectrum with high trans-mission efficiency. Prism-based imaging is compatible with different microscopy modalities from conventional (multicolor) fluorescence, single-particle tracking and super-resolution mi-croscopy (SOFI, SIM) to label-free quantitative phase imaging. We are developing and apply-ing open-source multiplane imaging hardware and software to facilitate widespread adoption.
Label-free imaging - Fourier-filtering quantitative phase imaging
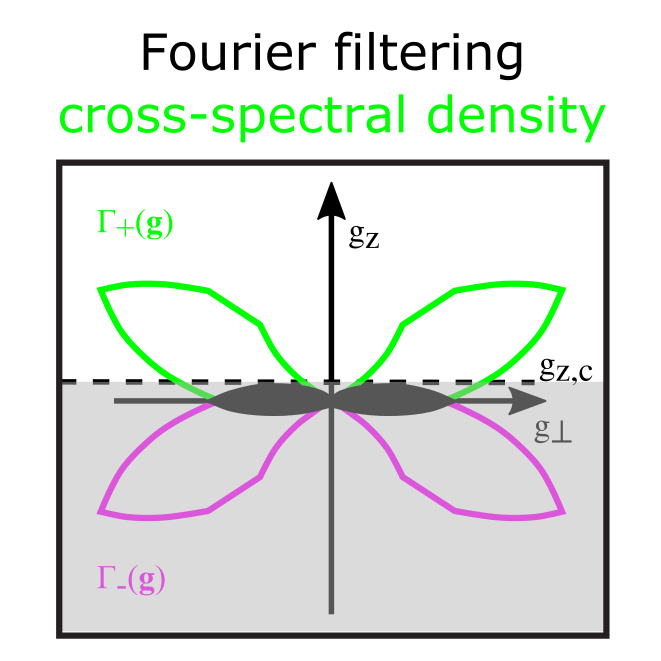
Label-free optical methods such as quantitative phase imaging (QPI) can be used to access mechanical properties and changes in (intra-)cellular morphology non-invasively. The measurements provide information about the local thickness and refractive index at high imaging speed. This allows e.g. long-term monitoring of cell dry mass, enabling measurements of cell growth rate and mass transport. We are using quantitative phase retrieval by Fourier cut-off filtering of a partially coherent white-light trans-illumination image stack. In combination with multiplane imaging, we can acquire 3D QPI data only limited by the camera speed ena-bling measurements with a vast range of timescales from milliseconds to days.
Fluorescence super-resolution imaging
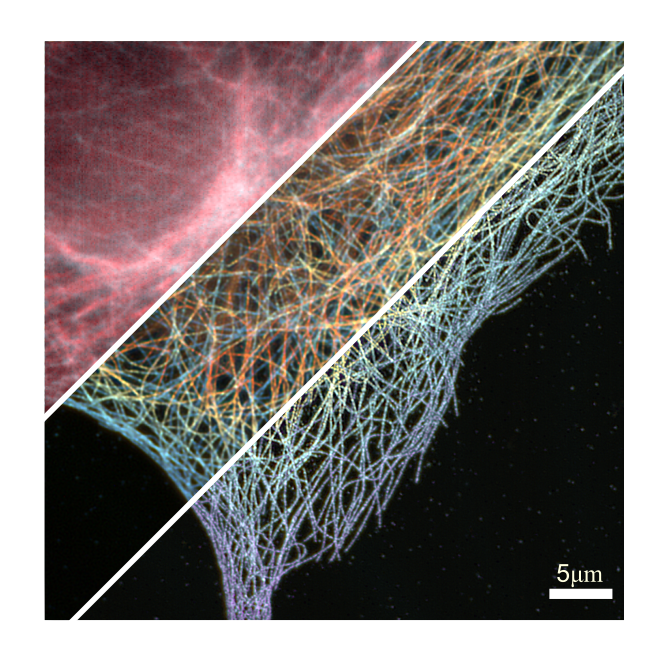
Fluorescence super-resolution provides molecular specificity and information below the diffraction limit. Our main interests are single-molecule based techniques that exploit fluores-cence blinking such as single-molecule localization microscopy (SMLM including PALM, STORM and PAINT) and super-resolution optical fluctuation imaging (SOFI). SMLM offers a high resolution in 3D, typically down to 10-50 nm. SOFI uses higher-order statistics that lead to a more moderate resolution gain, but instead has relaxed requirements on fluorophore pho-toswitching and sample properties. We have introduced prism-based 3D SOFI, multicolor im-aging based on spectral cross-cumulants and are investigating new classes of fluorophores for SOFI. We are also interested in new strategies for multiplane structured illumination to allow fast 3D imaging at minimal phototoxicity. We develop microscopes, establish labeling and im-age processing to enable e.g., quantification of protein clusters & complexes and identification of protein interactions.
Instruments
We are installing a multiplane microscope for ultrafast 3D data acquisition (fluorescence with SOFI/SIM and phase microscopy). We are also building a state-of-the-art super-resolution setup capable of 3D single-molecule localization microscopy and single-particle tracking. Both instruments will be compatible with live cell measurements. More details will follow soon. To discuss collaboration projects please contact Kristin Grußmayer. We are looking forward to working with you on quantitative imaging!