How Cytoskeletal Crosstalk Makes Cells Strong
Project Description
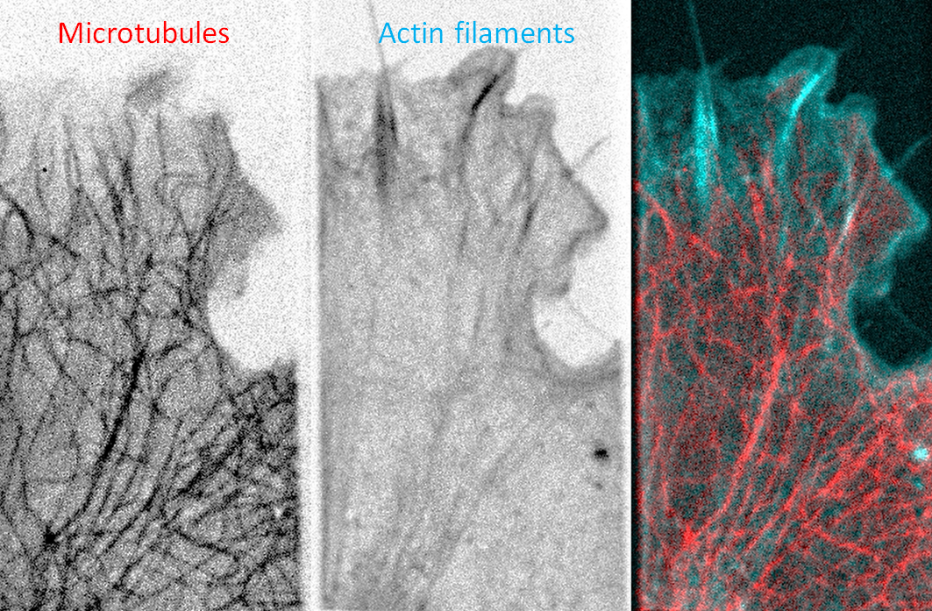
Our cells combine a superior mechanical strength with the ability to dynamically deform themselves during cell division and migration. They owe this paradoxical mechanical behavior to their cytoskeleton, composed of actin, microtubules, and intermediate filaments. These three filaments have traditionally been regarded as independent systems with separate cellular tasks. However, recent evidence suggests that they function in a coupled manner through physical interactions and biological regulation. We take a systems view on cell mechanics by asking how the composite nature of the cytoskeleton enables the cell to balance the opposing demands of mechanical strength and deformability. We study the collective mechanical properties of synthetic cytoskeletal networks reconstituted from purified components to understand how physical interactions generate mechanical synergy. We are also developing a technique to image force propagation inside these networks based on molecular force sensors. In parallel, we develop methods to perform mechanical measurements on single cells, to study the combined effects of physical interactions and biological regulation in cytoskeletal teamwork.
Contact: Michal Shemesh, Irene Istúriz Petitjean
Collaborations:
- Prof. Sarah Koester (Gottingen University, Germany)
- Prof. Anna Akhmanova (Utrecht University, Netherlands)
- Prof. Marileen Dogterom (Delft University of Technology)
- Prof. dr. E.M. (Elly) Hol (UMC Utrecht)
- Sandrine Etienne-Manneville (Institut pasteur )
Representative Publications:
- D. van de Willige, J.J.A. Hummel, C Alkemade, O.I. Kahn, F.K.C. Au, R.Z. Qi, M. Dogterom, G.H. Koenderink, C.C. Hoogenraad, A. Akhmanova, Gas2L1 regulates axon morphology through microtubule-modulated actin stabilization, EMBO Reports 20: e47732 (2019)
- Aufderhorst-Roberts, H.H. Koenderink, Stiffening and Fluidization in Vimentin Intermediate Filament Networks, Soft Matter 15:7127-7136 (2019)
- M. Preciado Lopez, F. Huber, I. Grigoriev, M.O. Steinmetz, A. Akhmanova, G.H. Koenderink, M. Dogterom, Actin-microtubule coordination at growing microtubule ends, Nat. Comm. 5: 4778 (2014)