Septin’s Roles in Cell Division
Project Description
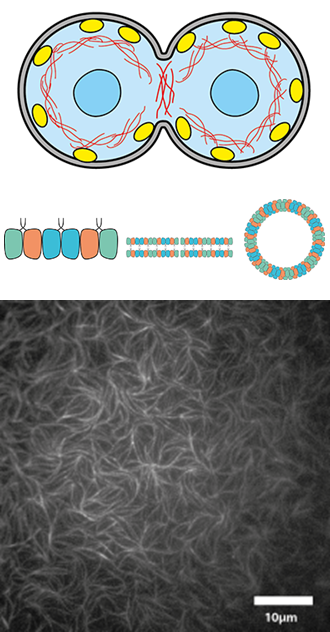
Traditionally, the cytoskeleton of animal cells is thought to consist of 3 filament types known as actin filaments, microtubules and intermediate filaments. However, recently septins have been discovered as the fourth component of the cytoskeleton. Septins are essential for cell division, host-pathogen interactions, vesicle trafficking and many other cellular functions. Vital for these functions is their ability to form higher order structures such as filaments, bundles, rings, and meshes that are able to interact with the cell membrane, microfilaments and microtubules. In humans, cells contain four subfamilies of septins that can form functional hexamers or octamers in a combinatorial fashion. These oligomers are then the building block to form the apolar filaments and higher order structures. We study the mechanism by which septin oligomers polymerize and interact with biomimetic cell membranes and with cytoskeletal filaments, and how they direct cell division. We use fluorescence and electron microscopy, atomic force microscopy, and quartz-crystal microbalance with dissipation (CQM-D) measurements of septin-membrane binding.
Contact: Gerard Castro Linares
Collaborators:
- Dr. Manos Mavrakis (Institut Fresnel, Marseille, France)
- Dr. Aurelie Bertin (Institut Curie, Paris, France)
- Dr. Pascal Verdier-Pinard (INSERM, Marseille, France)
- Dr. Ralf Richter (University of Leeds, UK)
Representative publications:
- M. Mavrakis, Y. Azou-Gros, F.-C. Tsai, J. Alvarado, A. Bertin, F. Iv, A. Kress, S. Brasselet, G.H. Koenderink, Thomas Lecuit, Septins promote F-actin ring formation by cross-linking actin filaments into curved bundles, Nature Cell Biology 16: 322-334 (2014)
- Chapter 18: Purification of recombinant human and Drosophila septin hexamers for TIRF assays of actin–septin filament assembly, M. Mavrakis, F.C. Tsai, G.H. Koenderink, in: Septins, Methods in Cell Biology 136 (August 2016)/Edited by A.S. Gladfelter, Amsterdam: Elsevier