The physics of cell division studied through synthetic cells
Marcos Arribas Perez
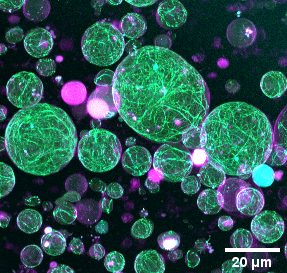
The division of eukaryotic cells is driven by a contractile ring which interacts with the plasma membrane and constricts it at the mid-cell. This ring is mainly formed by a cross-linked network of actin filaments anchored to the plasma membrane and myosin-II motor proteins which drive the constriction of the ring. However, the mechanisms of ring formation and action are still not well understood. So far, most efforts have been directed towards the understanding of how the actin cytoskeleton induces changes in the membrane shape. Nevertheless, the organisation and functionality of actin bundle networks confined within synthetic cells are also influenced by their binding to the membrane, the geometry of the compartment and the local curvature and mechanical properties of the membrane. Hence, we aim to investigate the role of the membrane geometry in the assembly and constriction of the actomyosin ring. For this purpose, we will reconstitute a minimal machinery required for actin ring assembly inside lipid vesicles and use diverse physical and biochemical strategies, including microfluidic devices, optical tweezers, curvature inducing proteins and DNA nanostructures, to reshape the synthetic cell geometry in a controlled manner and analyse the dynamic response of the actin networks to those changes in the shape of the compartment.