Hydrogen adsorption on carbon nanotubes, activated carbons
In the past record hydrogen storage capacities were reported for the surface adsorption on or inside carbon nanotubes. Such reports triggered us to compare nanotubes, activated carbons and nano fibres with each other. The results indicate that only moderate adsorption capacities of a few wt.% can be realised and that the fundamental attraction, based on weak van der Waals interactions, is comparable for the different types of carbons. At least, this holds for the majority of the hydrogen adsorbed. If one searches for preferential sites for adsorption one can deduce, based on inelastic neutron scattering data, that on sites where a hydrogen molecule touches more than one carbon surface at the same time the interactions are larger. However, the interactions are still too low to effectively bind the hydrogen in this relatively limited number of ‘groove sites’.
The molecular dynamics animation below illustrates what happens with hydrogen adsorbed in a groove between two adjacent nanotubes when the temperature is raised to 80K. The hydrogen easily diffuses across the surface of the tubes and then escapes to the gas phase for most of the time.
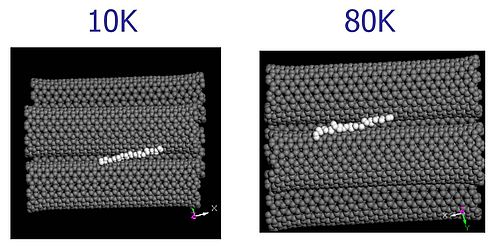
[AVI @ 10K]
[AVI @ 80K]
Published work
- H.G. Schimmel, G.J. Kearley, M.G. Nijkamp, C.T. Visser, K.P. de Jong, F.M. Mulder; Hydrogen adsorption in carbon nanostructures: comparison of nanotubes, fibers, and coals, Chem. Eur. J. 9 (2003) 4764-4770. [PDF]
- - H.G. Schimmel, G. J. Kearley, F. M. Mulder, Resolving rotational spectra of hydrogen adsorbed on a single walled carbon nanotube substrate, ChemPhysChem 5 (2004) 1053. [PDF]
- - H.G. Schimmel, M.G. Nijkamp, G.J. Kearley, A. Rivera, K.P. de Jong and F.M. Mulder; Hydrogen adsorption in carbon nanostructures compared; J. Mater Scie. Eng B. 108 (2004) 124-129. [PDF]