Fluoride Ion Batteries
Fluoride Ion Batteries are a novel, alternative battery chemistry based on F- anions as a charge carrier. They are promising as a safer and more sustainable option to their lithium counterpart, due to the absence of a liquid and flammable electrolyte and the use of abundant and globally available fluoride ions (Fˉ).
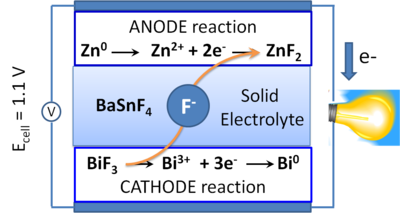
In this ion shuttle battery concept, energy is stored and released by conversion reactions at the electrodes, which are based on oxidation and reduction of a metal and metal fluoride, respectively.
Given the fact that multiple electrons can be stored by a single metal atom in electrodes based on conversion reactions, this battery chemistry holds promise for high energy densities. For example, a theoretical volumetric energy density in the range of could be provided by a combination of CuF2 as cathode and Sm as anode, which amounts to 8x the theoretical values for materials used in conventional Li-ion batteries.
In our group, we explore multiple aspects of the solid-state chemistry of metal fluorides to tune the properties of solid electrolytes and active electrode materials envisioned for Fluoride Ion Batteries.
While the high energy densities promised for this concept may lead to considerable increase in maximum ranges for electric transportation, a combination of cheap and abundant metals may enable widespread use of Fluoride Ion Batteries in stationary applications. Therefore, it is mandatory to take sustainability concepts in the design of new materials for this battery chemistry, as well as focus research efforts into widely available and abundant metal elements as potential active electrode materials. The key challenges lie on decreasing capacity fading caused by large volume changes during charge/discharge cycles as well as improving rates for the corresponding electrochemical reactions.
Furthermore, the ion transport between electrodes can be realised by either liquid or solid electrolytes, with the use of superionic conductors playing an important role for the latter in current research and future applications. Current challenges are based on designing solid electrolytes for F- ions that exhibit high ionic conductivities at room temperature whilst preserving a broad electrochemical stability window.
We employ a broad range of advanced characterization methods, such as neutron diffraction and solid-state nuclear magnetic resonance spectroscopy in order to gain insight on structure-property relations governing the function of metal fluorides in Fluoride Ion Batteries. The use of these advanced characterisation tools for ex situ, in situ and operando studies of metal fluorides in electrochemical cell is expected to provide a deeper understanding of the causes for current limitations of this battery concept, such as small currents and low cyclability, and offer viable mitigation strategies. Furthermore, this experimental approach is supported by quantum chemical computations based on density functional theory and molecular dynamics to understand phase stabilities and predict pathways for ion transport across different metal fluoride structures.
Through this research effort we expect to unleash the potential of Fluoride Ion Batteries as a sustainable battery chemistry geared towards widespread application in the decarbonized energy landscape of the coming decades.