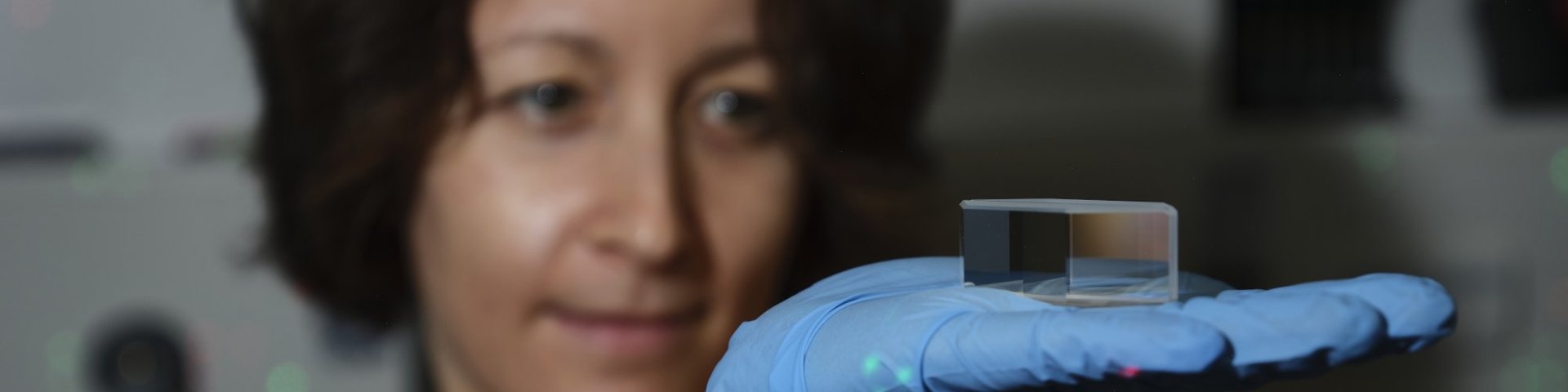
Kristin Grußmayer pushes the boundaries of microscopy with new techniques to study the exact location of molecules, and to make extremely fast 3D images of the cell. Her research group will take a look at the early stage of Huntington’s disease on a very small scale, now possible thanks to these advanced microscopy methods: “One of the biggest challenges in the super resolution field is how to work with living cells while making sure they stay happy.”
Kristin Grußmayer, who set up her lab at TU Delft in February 2021, is a physicist by training but she was always interested in biology as well: “I look from a physicist perspective how to work with biological components”, Grußmayer says. “You need knowledge from different disciplines to apply microscopy to biology: physics, a bit of math, biology and chemistry has to come together. With my group at the Bionanoscience department I explore super resolution microscopy methods based on single molecules. I develop these methods to figure out new things about how cells work.”

I find that people who want to work with a new method of microscopy need to have patience and not give up too soon, because the benefits when you get it to work can be big.
Kristin Grußmayer
Three-dimensional look at the cell
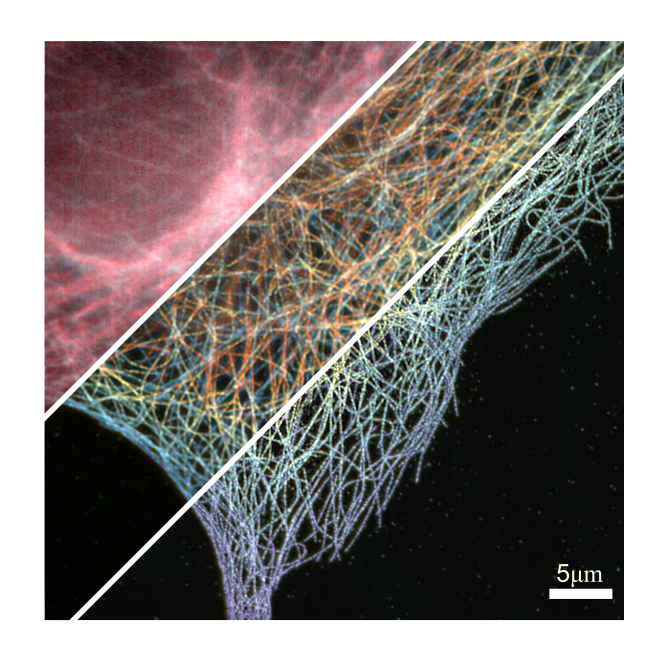
The heart of Grußmayer’s work is about pushing the boundary of microscopy methods, in order to ultimately apply them in life science. What is special about the kind of microscopy that Grußmayer does? “Our microscopy is compatible with living cells and it can show the inner workings of cells in three dimensions”, Grußmayer explains. “We use different techniques to get more information. To make specific molecules visible under the microscope, we can label them with contrasting fluorescent dyes. Then, we can turn individual dyes “on” and “off”. This allows us to determine the positions of the molecules very precisely, and to obtain a super-resolution image by adding all locations. We also use statistics to analyse the relations in the emitted light. And with our new method to retrieve quantitative phase images, we only rely on the optical properties of the cells themselves. We can now figure out more about the dynamics inside the cell at unprecedented speed without labeling the molecules.”
With a colleague, Grußmayer also implemented a new multi-plane microscope, which can now take eight images in one snapshot: “By combining these images we get a 3D image of the cell. This way, we look at for example the morphology of the cell, and we can study how the cellular mass is moving around in the cell. My first PhD student in Delft just finished building a new version of such a system.”
Figuring out Huntington’s disease
Grußmayer collaborates with neurobiologist Hilal Lashuel and his group from EPFL (École Polytechnique Fédérale de Lausanne), the technical university in Switzerland where she did her postdoc, to study Huntington’s disease: “As with most neurodegenerative diseases, we don’t know what the underlying molecular mechanism is that leads to the disease. In the coming years we will look at the early phase of Huntington’s on a very small scale. With our advanced microscopy we can quantify the dynamics of the protein buildup that forms when people have Huntington's disease: how do the proteins start to accumulate and clump together and how do they behave later on in the course of the disease?”
How to keep the cells happy
Huntington’s disease is caused by a defect in a single gene, which Grußmayer introduces in a cell to model Huntington’s disease: “We label the proteins to look at the living cells in high resolution – for this we are developing new microscopy labels together with chemists Richard Wombacher and Kai Johnsson at the Max Planck Institute in Heidelberg. We complement super-resolution techniques with phase microscopy, to get a more general sense of what the cell is doing. Then we combine the information from these different imaging techniques.”
“I want to use machine learning to figure out how we can efficiently switch between these techniques: which method should we use when, and how can we still be gentle to the cell so it remains happy? This is one of the biggest remaining challenges in the super resolution field: working with living cells at high resolution, while making sure the cells stay happy – and the data is reliable.”

Implementing new microscopy methods
Grußmayer explains how she manages to implement her new techniques more and more in Delft: “TU Delft is really big in imaging and microscopy. Within our Bionanoscience department for example, I work with Gijsje Koenderink on how cytoskeleton elements interact, which is a great way to apply our microscopy methods. I am also in constant exchange with image processing specialists at Imaging Physics and the Department of Precision and Microsystems Engineering. I find that people who want to work with a new method of microscopy need to have patience and not give up too soon, because the benefits when you get it to work can be big.”
Open science
Together with a team of researchers, Grußmayer was recently awarded one of the TU Delft AI labs: “I am happy to start this project into deep learning and smart super-resolution microscopy.” Grußmayer is an advocate for open science. “When we come up with new microscopy methods, we share them on our lab homepage as well as on GitHub for open science, so that others can build these methods as well.” She notes that there are so few female scientists in the field of physics. “This has improved since I started studying, but there is still a long way to go. I hope that I can be a role model.”