A longer life in good health? The end of genetic disorders? Crops that are able to survive in the harshest conditions? CRISPR-Cas9 brings all of this and more within our grasp. The research group of Dr Stan Brouns at the department of Bionanoscience is conducting fundamental research into how CRISPR systems function. What is his take on the forthcoming revolution?
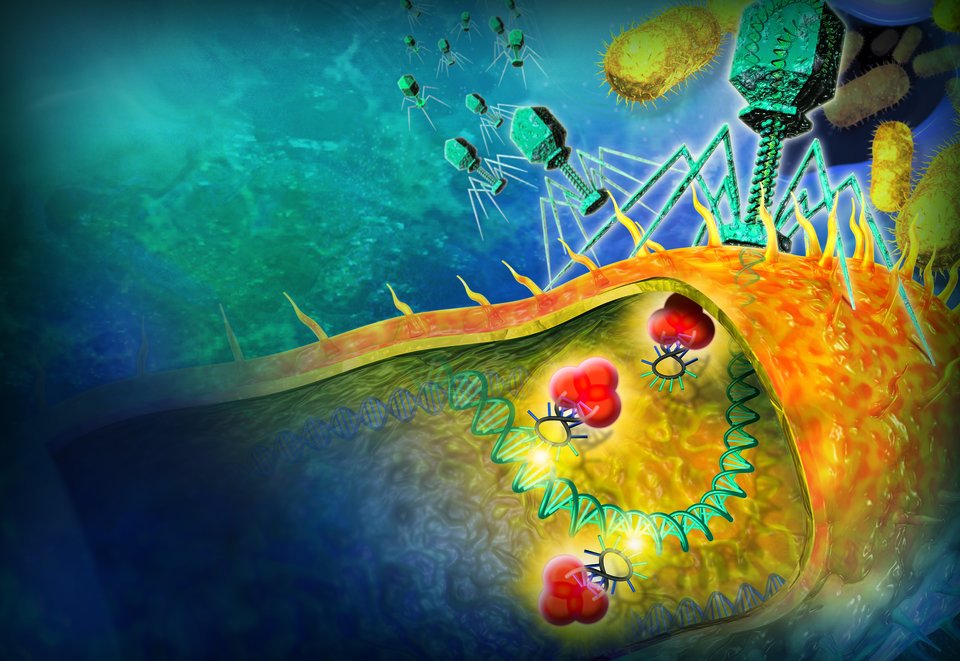
“Look, this is a Cascade complex,” says Dr Brouns, turning a strange-looking lump of plastic over in his hands. The object most resembles a chunk of coral, with a contorted and uneven surface. It is, in fact, a model of a cluster of proteins that are to be found in the cytoplasm of certain bacteria. “This protein here,” he points, “is the spine of the complex. And see these blobs here? Those are also proteins. All of them are essential, otherwise the system doesn’t work.”
Molecular scissors
What is the function of this particular cluster of proteins? Put simply, it is an essential weapon that bacteria use to exterminate viruses, which are professional killing machines that have been bacteria’s nemesis since the dawn of evolution. For example, every day, simple viruses known as ‘bacteriophages’ kill one third of all the bacteria in the oceans. “The virus injects its DNA or RNA into a cell in order to try to take it over,” explains Dr Brouns. “And if this hijacking is successful, the virus is able to use that cell as a little factory to produce copies of itself.” A strategy as simple as it is deadly.
Dr Brouns’ protein cluster, the Cascade complex, leaps into action the moment a virus injects its genetic material. It seeks out the virus’s DNA and clamps onto it tightly. It then sends a signal to another protein, which comes along and cuts the virus to ribbons using a pair of molecular scissors. Dr Brouns is investigating how this type of bacterial defence system functions.
Mysterious code
How does the Cascade complex distinguish between viral DNA and the bacterium's own DNA? That is where something known as CRISPR comes in: ‘Clustered Regularly Interspaced Short Palindromic Repeats’. Researchers discovered these constantly repeating sequences of code (‘repeats’) in the DNA of the well-known E. coli bacteria in the 1980s. “Back then, nobody knew what the sequences meant,” says Dr Brouns. “It was only in 2005 that it was recognised that the DNA segments located between the repeats corresponded with the DNA of viruses.”
The DNA of viruses? Yes, indeed. Sometimes a bacterium survives an attack by a virus, for example because the virus was weakened. In those rare cases, the bacterium seizes a trace of the virus’s DNA. By ‘unzipping’ its own DNA at the location of the repeats, the bacterium then builds the virus’s DNA into its own genetic code. The effect is comparable to how a vaccine works. From that moment on, the bacterium has that virus etched into its memory forever. If that same type of virus is foolish enough to try attacking that bacterium again, it recognises its assailant and can dispatch a cutting protein, such as the Cascade complex. Using two strands of RNA as a ‘cheat sheet’, the protein cluster goes on the hunt for enemy DNA. And if the complex finds a match, it is simply a matter of grip, snip and away.
Cutting and pasting
But CRISPR systems such as the Cascade complex are much more than just a biological curiosity. Scientists have been able to use them to edit DNA since the autumn of 2012, when Professors Jennifer Doudna (UC Berkeley, USA) and Emmanuelle Charpentier (then at Umeå University, Sweden) succeeded in reconstructing the CRISPR system of a streptococcus bacteria that uses the Cas9 protein. The two scientists unravelled the bacteria’s defence mechanism. Then, one of Prof. Doudna’s post-doc researchers managed to combine the two RNA stands into a single ‘guide RNA’ (the cheat sheet alluded to earlier), which he could programme however he wished. This made it possible to use any arbitrary segment of DNA as a guide RNA and therefore to cut DNA wherever required.
Of course, rewriting DNA is quite a different challenge than merely cutting it apart. But further research also proved the latter to be possible using CRISPR-Cas9. Researchers are now able to send a segment of DNA along with the Cas9 protein. As soon as the protein snips through a cell’s DNA, the cell tries to repair the strand as quickly as possible. If the ends of the accompanying DNA segment fit with the ends of the cut strand, the cell will use that genetic material to repair the strand. Researchers are able to place other nucleotides (and even complete genes) between the ends of the accompanying DNA segment.
Aggressive white blood cells
CRISPR-Cas9 is now being used by thousands of research teams around the world to very precisely deactivate or overwrite particular genes. This technology has enormous potential. “One specific example is a clinical trial that is currently in progress in which white blood cells extracted from cancer patients are being modified using Cas9 to be more aggressive,” says Dr Brouns. “The hope is that this will enable the modified white blood cells to get rid of tumours on their own.” It is just one example of the numerous possible applications of CRISPR-Cas9.
Naturally, the technology has certain limitations. “Gene editing in living creatures, for example, is a challenge,” he notes. Particularly when trying to introduce changes in tissue that is rarely regenerated. Consider, for example, Duchenne Muscular Dystrophy (DMD), which damages and weakens the muscles. “Setting aside the ethical objections for a moment,” says Dr Brouns, “in theory you could deactivate this disease at the embryonic stage. Or you could select embryos that are unaffected by this disorder, which already happens in clinics. But once a person is born with this disease, it is actually too late.” Still, scientists have already made significant strides towards a cure. “It is possible to embed a repair sequence into a specific virus and inject that virus into a mouse with DMD. The virus will then disseminate the new genetic material.” And the outcome of this procedure? Mice with stronger muscles. But unfortunately not as strong as those of healthy mice, as yet.
A kit full of tools
What is not widely known is that there are considerably more CRISPR systems in existence than merely the variant that uses the now familiar Cas9 protein. And the functioning of one can be quite unlike the next. For example, instead of a straight cut, one type of protein makes an angular incision in the DNA into which the repair material fits like a puzzle piece, which increases the cell’s chances of successfully repairing its DNA. Furthermore, the size of the Cas proteins varies in each system. Cas9 is relatively large and is therefore not able to operate in every type of cell.
Another type of cutting enzyme, known as Cpf1, is also looking promising. “Cpf1 is going to be quite successful as well,” predicts Dr Brouns. “It has a tiny RNA that is capable of cutting through both DNA strands. And since it comprises only 40 nucleotides, compared to Cas9's 100, it is cheaper to produce. Moreover, it makes it easier to cut in multiple locations simultaneously.”
In short, CRISPR-Cas is not a single tool, but rather a whole kit full of different kinds of implements. Where one job requires a screwdriver, another requires a hammer. And the good news is, new tools are being added to the kit all the time. Hopefully these new discoveries will eventually lead to a point where scientists can mould all extant cells to their will.
Breaking new ground
And where will all this end? The first children with a modified DNA have already been born. And while we are at it, will we choose to make these genetically modified children more intelligent? With violet eyes? And maybe add some sweet little dimples to their cheeks? “Yes, well,” says Dr Brouns, “at a certain point you stray beyond the bounds of diseases into areas that people find desirable. That is something we have to consider with great care.”
Brouns knows, of course, that it is becoming more and more pressing to answer questions of this nature. However, his goal is to unravel the biology behind CRISPR-Cas systems in order to figure out down to the minutest detail how these extraordinary defence mechanisms function. One question that the Brouns group is now trying to answer, for instance, is why bacteria seem to prefer taking certain strands of a virus’s DNA to build into their own DNA, as opposed to others. These endeavours will keep Brouns and his research group occupied for some time yet. “We have already learned so much, yet I expect that there are many microbial defence mechanisms still to discover.”
But the most striking thing remains just how ingenious the CRISPR systems are, which is precisely why they are a source of fascination. “Every time we look at them, their functioning always seems more complex than we first thought,” says Dr Brouns. “They never cease to amaze us.”