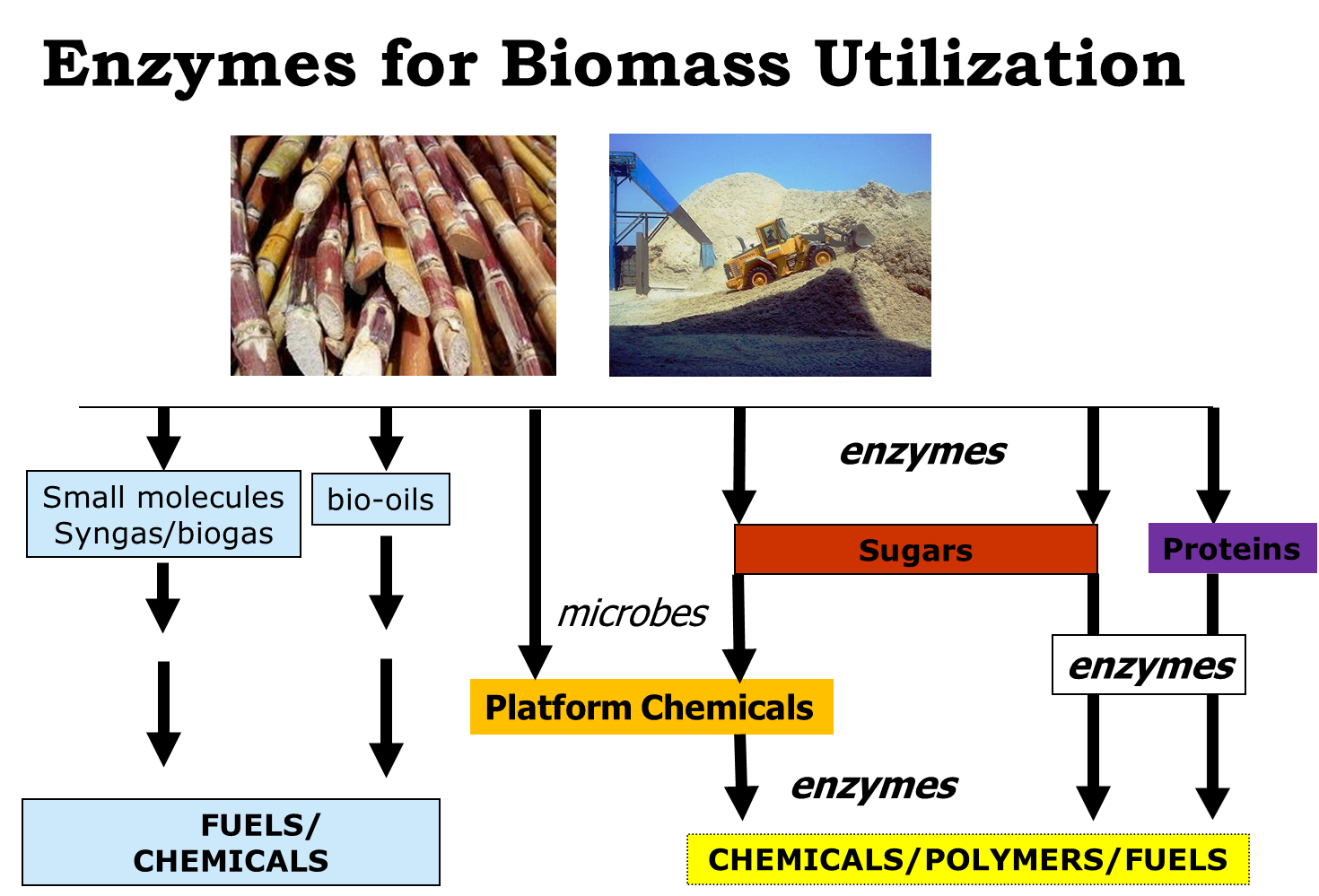
Nature uses enzymes to catalyze a wealth of biotransformations. However in practice these transformations are carried out in-vivo under conditions which are far from suitable for large scale production of chemicals. It is our challenge to engineer or design enzymes in such a way that they can be employed in-vitro as catalysts.1 Different classes of enzymes can be employed as highly promising catalysts for a variety of reactions that are pivotal for the conversion of biobased molecules. Some notable examples from our lab are the reduction of volatile fatty acids using Pyrococcus furiosus strains,2 the oxidation of alcohols using a variety of enzymes and cofactor regeneration methods,3 enzymatic reductions using water as electron donor,4 and the hydration of oleic acid leading to i.e. polymeric building blocks.5,6
- F. Hollmann, I.W.C.E. Arends, K. Buehler, A. Schallmey and B. Bühler, Enzyme-mediated oxidations for the chemist, Green Chem. 13 (2011) 226-265.
- Y. Ni, P.L. Hagedoorn, J-H. Xu, I.W.C.E. Arends and F. Hollmann, Pyrococcus furiosus-mediated reduction of conjugated carboxylic acids: towards using syngas as reductant, J. Mol. Catal. B: Enzymatic 103 (2014) 52-55.
- D. Holtmann, M.W. Fraaije, I.W.C.E. Arends, D.J. Opperman and F. Hollmann, The taming of oxygen: biocatalytic oxyfunctionalisations, Chem. Commun. 50 (2014) 13180-1320.
- M. Mifsud, S. Gargiulo, S. Iborra, I.W.C.E. Arends, F. Hollmann and A. Corma, Photobiocatalytic chemistry of oxidoreductases using water as the electron donor, Nat. Commun. 5:3145 (2014) 1-6.
- A. Hiseni, L.G. Otten and I.W.C.E. Arends, Identification of catalytically important residues of the carotenoid 1,2-hydratases from Rubrivivax gelatinosus and Thiocapsa roseopersicina, Appl. Microbiol. Biotechnol. 100 (2016) 1275-1284.
- A. Todea, A. Hiseni, L.G. Otten, I.W.C.E. Arends, F. Peter and C.G. Boeriu, Increase of stability of oleate hydratase by appropriate immobilization technique and conditions, J. Mol. Catal. B: Enzymatic 119 (2015) 40-47.