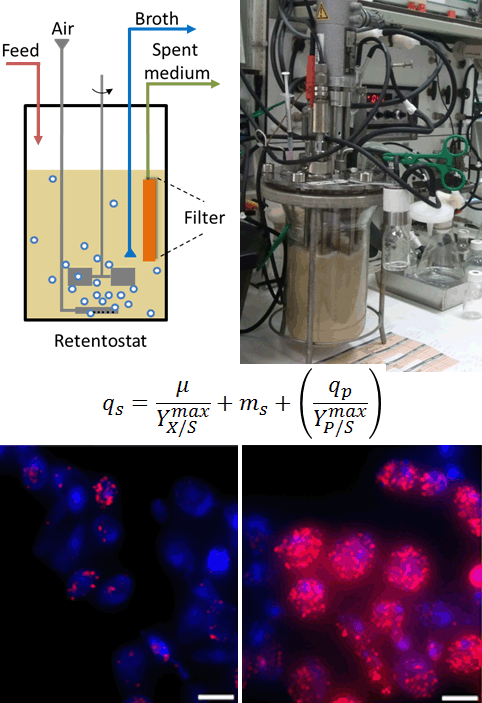
Uncoupling growth from product formation
Microbial biotechnology plays a pivotal role in providing sustainable alternatives for petrochemical production of a large variety of compounds. Product formation by microorganisms is, however, typically coupled to growth. Since growth competes with product formation for raw materials, microbial biomass is not only the catalyst, but also an undesirable by-product in many processes. Non-dividing but metabolically active cells are of great interest for biotechnological applications. Yet, this physiological status is difficult to achieve. Using advanced, continuous cultivation techniques called retentostats, enable to capture cells in such a state. Using retentostat, we explore quantitatively the physiology of yeast cells and the possibility to uncouple growth from product formation.
Retentostat, in which yeast cells do not divide but survive for weeks also offer an excellent model to explore chronological aging and identify mechanisms relevant for metazoan.
Collaborations
Funding
People involved
Related publications
Bisschops, M. M., M. A. H. Luttik, A. Doerr, P. J. T. Verheijen, F. Bruggeman, J. T. Pronk, P. Daran-Lapujade. 2017. Extreme calorie restriction in yeast retentostats induces uniform non-quiescent growth arrest. BBA Mol.Cell Res. 1864:231.
Rebnegger, C., T. Vos, A. Graf, M. Valli, J.T. Pronk and P. Daran-Lapujade, and D. Mattanovich. 2016. Pichia pastoris exhibits high viability and low maintenance-energy requirement at near-zero specific growth rates. Appl.Env.Microbiol. 82:4570.
Vos, T., H.D.V. Hakkaart, E.A.F. de Hulster, A.J.A. van Maris, J.T. Pronk, and P. Daran-Lapujade. 2016. Maintenance-energy requirements and robustness of S. cerevisiae during aerobic cultivation at near-zero specific growth rates. Microb.Cell Fact. 15:111.
Bisschops, M.M.M., T. Vos, R. Martínez-Moreno, P. de la Torre Cortés, J.T. Pronk, and P. Daran-Lapujade. 2015. Oxygen availability strongly affects chronological lifespan and thermotolerance in batch cultures of Saccharomyces cerevisiae. Microbial Cell. 2:429-444.
Ercan, O., M. M. Bisschops, W. Overkamp, T. R. Jørgensen, A. F. Ram, E. J. Smid, J. T. Pronk, O. P. Kuipers, P. Daran-Lapujade, and M. Kleerebezem. 2015. Physiological and transcriptional responses of different industrial microbes at near-zero specific growth rates. Appl.Env.Microbiol. 81:5662-5670.
Vos, T., P. de la Torre Cortes, W. M. van Gulik, J. T. Pronk, and P. Daran-Lapujade. 2015. Growth-rate dependency of de novo resveratrol production in chemostat cultures of an engineered Saccharomyces cerevisiae strain. Microb.Cell Fact. 14:133.
Bisschops, M. M., P. Zwartjens, S. G. Keuter, J. T. Pronk, and P. Daran-Lapujade. 2014. To divide or not to divide: a key role of Rim15 in calorie-restricted yeast cultures. BBA Mol.Cell Res. 1843:1020-1030.
Binai, N. A., M. M. Bisschops, B. van Breukelen, S. Mohammed, L. Loeff, J. T. Pronk, A. J. Heck, P. Daran-Lapujade, and M. Slijper. 2014. Proteome adaptation of Saccharomyces cerevisiae to severe calorie restriction in retentostat cultures. J.Proteome Res. 13:3542-3553.
Boender, L. G., M. J. Almering, M. Dijk, A. J. van Maris, J. H. de Winde, J. T. Pronk, and P. Daran-Lapujade. 2011. Extreme calorie restriction and energy source starvation in Saccharomyces cerevisiae represent distinct physiological states. BBA Mol.Cell Res. 1813:2133-2144.
Boender, L. G., A. J. van Maris, E. A. Hulster, M. J. Almering, I. J. Klei, M. Veenhuis, J. H. Winde, J. T. Pronk, and P. Daran-Lapujade. 2011. Cellular responses of Saccharomyces cerevisiae at near-zero growth rates: transcriptome analysis of anaerobic retentostat cultures. FEMS Yeast Res. 11:603-620.
Boender, L. G., E. A. de Hulster, A. J. van Maris, P. Daran-Lapujade, and J. T. Pronk. 2009. Quantitative physiology of Saccharomyces cerevisiae at near-zero specific growth rates. Appl.Env.Microbiol. 75:5607-5614.
