Proton therapy research
My research involves a range of proton and traditional radiation therapy topics, mostly focusing on novel treatment planning approaches. These include handling uncertainties (arising from e.g. patient positioning and proton range errors, breathing and organ motion), quantifying treatment robustness, developing robust optimization methods, optimizing radiotherapy fractionation and combined modality treatments, building decision support tools and exploring biology based treatment planning. Most projects are carried out in close collaboration with clinical partners, such as Erasmus Medical Center in Rotterdam of the Holland Proton Therapy Center right next door.
Specific ongoing projects are:
Robustness evaluation and robust optimization of treatment plans: Protons have a significantly better ability to deposit dose exactly at the tumor location while irradiating surrounding organs at risk (OARs) to a much lesser extent than photons. However, this better dose conformity comes at the price of increased sensitivity of proton treatments to uncertainties such as patient positioning or proton range. Proton plans therefore have to be tested for robustness against these uncertainties to assure their adequacy. Modern commercial treatment planning systems offer tools to perform robustness analyses for a limited number of potential error scenarios. However, with advanced uncertainty quantification methods (based on Polynomial Chaos Expansion) one could identify which error scenarios should be used in order to derive computationally affordable, yet meaningful and general metrics of robustness, that can approximate more precise probabilistic measures with sufficient accuracy. Furthermore, the direct incorporation of uncertainties into the treatment plan optimization itself (via robust optimization or probabilistic treatment planning) can produce plans that are much more resistant against uncertainties and at the same time virtually equally high quality as plans without considering uncertainties.
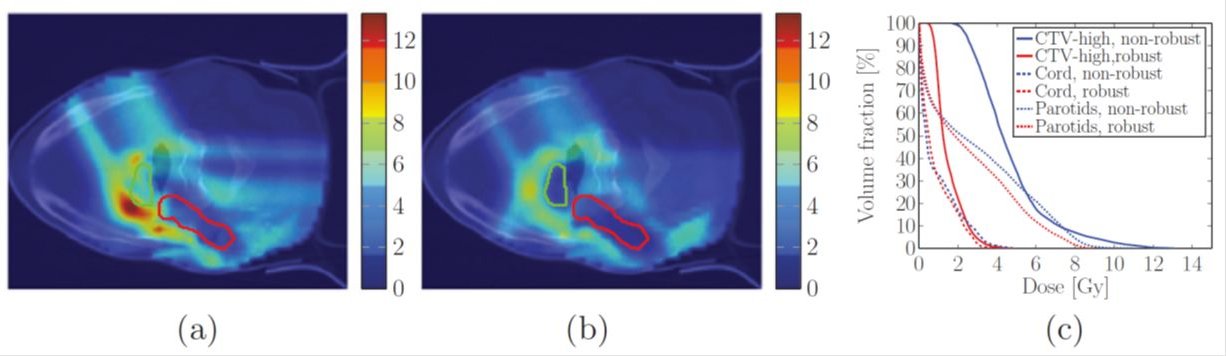
Fractionation optimization: Radiotherapy treatments are typically performed in numerous treatment sessions known as fractions. Historically, 25-35 fractions have been used over the course of 5-7 weeks, with one fraction being delivered every weekday. In recent years hypofractionated treatments – using fewer treatment sessions and higher doses per fraction - have also become more and more widespread. Currently the fractionation decision, i.e. how many treatment sessions to use and how much dose to deliver per fraction for a given patient is made by the physician, typically before treatment planning only based on clinical experience and anatomy. Hence fractionation is not yet optimized to provide the best outcome on an individual basis. Furthermore, with the spread of heavy ion particle therapy (proton, helium and carbon therapy in particular) an interesting question is how to use these scarcely available treatment modalities optimally. Thus, by optimizing the fractionation together with the spatial dose distribution the therapeutic ratio can be increased and the effectiveness of radiotherapy can further be advanced.
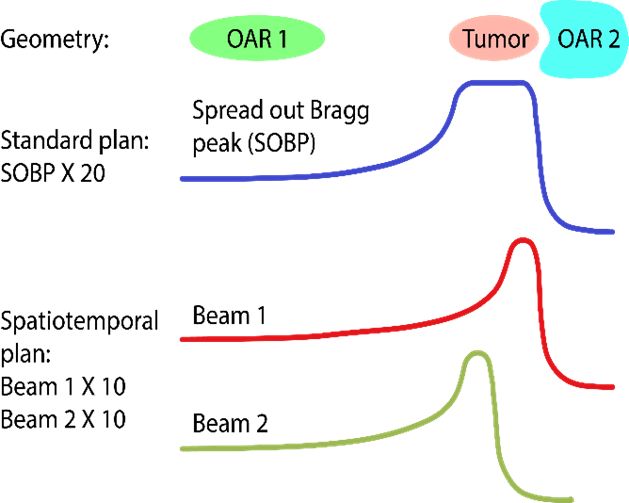
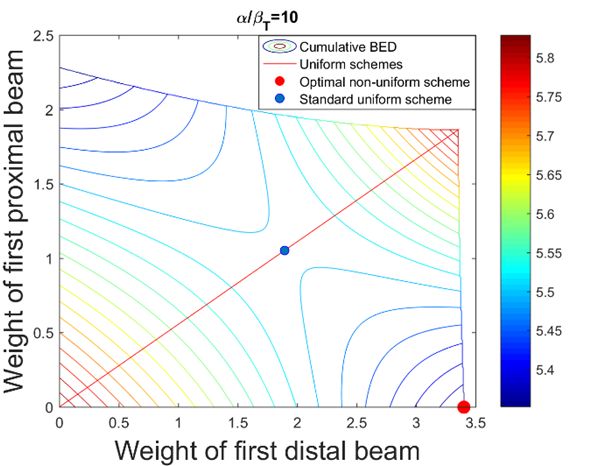
Spatiotemporal optimization: Cancer patients being treated with radiotherapy typically receive their treatment over the course of several weeks, during which dose is delivered in daily portions known as fractions. The rationale behind protracting the therapy is that healthy cells can typically recover from radiation damage better than cancer cells, therefore giving multiple smaller daily dose fractions instead of a few large doses (hypofractionation) increases the therapeutic ratio. It may be possible to design treatments where on different days, different parts of the tumor are treated with single high fraction doses, which ultimately may lead to better sparing of organs-at-risk than the current approach of delivering the same dose distribution in every fraction. This is a highly promising new technique known as spatio-temporal optimization, which can be especially useful for treatment sites where integral dose is limiting, such as liver or brain tumors.
References:
Z. Perkó, S. R. van der Voort, S. van deWater, et al., “Fast and Accurate Sensitivity Analysis of IMPT Treatment Plans Using Polynomial Chaos Expansion“, Physics in Medicine and Biology, 61 (12), pp. 4646-4664, (2016)
S. R. van der Voort, S. van de Water, Z Perkó, et al., "Robustness Recipes for Minimax Robust Optimization in Intensity Modulated Proton Therapy for Oropharyngeal Cancer Patients", International Journal of Radiation Oncology* Biology* Physics, 95 (1), pp. 163-170, (2016)
Z. Perkó, T. Bortfeld, T. Hong, et al., "Optimal Fractionation Schemes for Liver SBRT Based on BED", 18th International Conference on the use of Computers in Radiation Therapy, ICCR 2016, London, UK, (2016)
Z. Perkó, T. Bortfeld, T. Hong, et al., "BED Consistent Extrapolation of Mean Dose Tolerances", 58th Annual Meeting & Exhibition of American Association of Physicists in Medicine, AAPM 2016, Washington, DC, US, (2016)
J. Unkelbach, Z. Perkó, J. Wolfgang, et al., "Biological Dose Escalation for Liver SBRT Through Spatiotemporal Fractionation", 58th Annual Meeting & Exhibition of American Association of Physicists in Medicine, AAPM 2016, Washington, DC, US, (2016)