Magnesium-ion batteries
Li-ion chemistry today dominates the rechargeable battery market due to its excellent performance. Nevertheless, cost for large scale introduction of electricity storage systems to cope with the upcoming energy transition, remains an issue. Other chemistries are on the horizon, such as Sodium, Zink, Magnesium, and Calcium, which are much cheaper because of its large natural abundance on earth and the way these materials are recovered. In order to compete with the existing Li-ion batteries, the kinetics of these novel systems need to be unravelled to further improve the performance. The focus here will be on Mg-ion battery materials, which will be studied in either aqueous and non-aqueous electrolytes, but also solid electrolytes of ceramic, glassy and/or polymer origin are being developed.
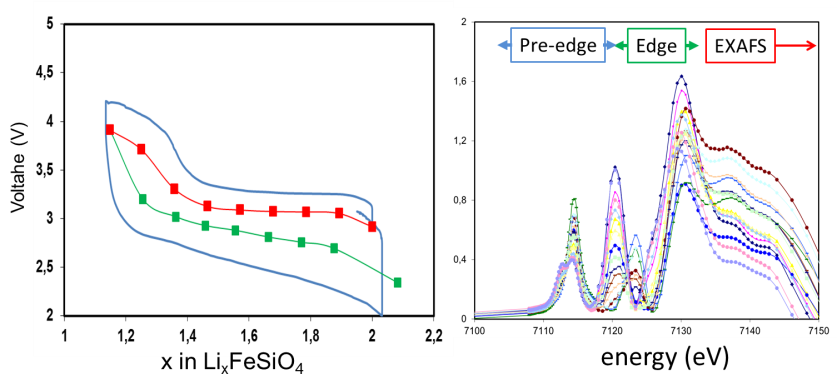
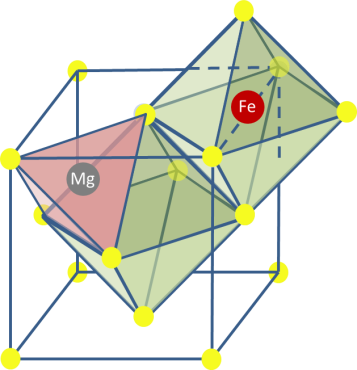
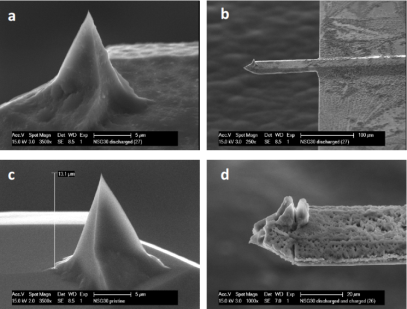
Within the Storage of Electrochemical Energy (SEE) group we investigate the fundamental properties of Mg-ion, including their phase transition mechanism and Mg-ion transport properties, both key properties that determine macroscopic battery performance. The techniques used to investigate these properties include operando X-ray and Neutron Diffraction, operando micro-beam diffraction, Solid State NMR, operando Neutron Depth Profiling, Density Functional Theory and Phase Field Modeling, operando Scanning Electrochemical Microscopy coupled to an Electrochemical Impedance Spectrometer, operando Atomic Force Microscopy. Besides, SEE has a strong link with the “DUBBLE’ beam line at the European Synchrotron Radiation Facility, where operando measurements with our electrochemical equipment is available.