Quantum mechanics and quantum technology 1.0
Quantum mechanics is the science of the very small. It explains the behavior of matter and its interactions with energy on the scale of atoms and subatomic particles.
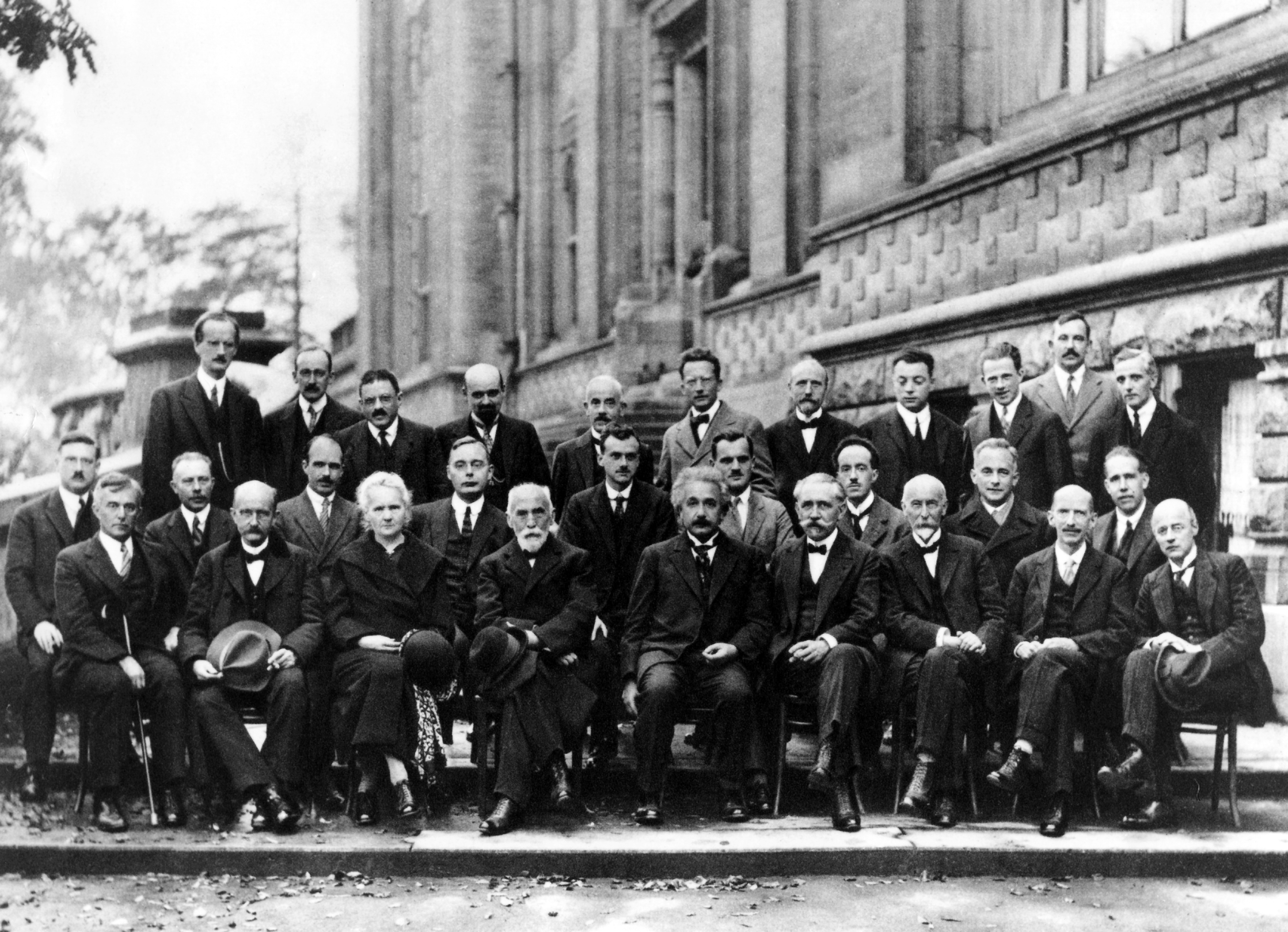
Our understanding of quantum mechanics accelerated in the beginning of the 20th century. At the Solvay International Conference on Electrons and Photons, organised in 1927 in Brussels, 29 prominent physicists discussed the basics of quantum theory and laid the foundation for today’s quantum mechanics. Seventeen of them already were, or would become, Nobel Prize winners. Amongst them were Albert Einstein, Marie Curie, Max Planck, and Niels Bohr.
The Solvay Conference in 1927 in Brussels, 5th council of physics
Back row L-R: A. Piccard, E. Henriot, P. Ehrenfest, E. Herzen, Th. de Donder, E. Schrödinger, E. Verschaffelt, W. Pauli, W. Heisenberg, R. Fowler, L. Brillouin
Middle row L-R: P. Debye, M. Knudsen, W.L. Bragg, H.A. Kramers, P.A.M Dirac, A. Compton, L. de Broglie, M. Born, N. Bohr
Front row L-R: I. Langmuir, M. Planck, M. Curie, H. Lorentz, A. Einstein, P. Langevin, C. E. Guye, C.T.R Wilson, O.W. Richardson
Many technologies we use in modern life could be invented because of our understanding of quantum mechanics: lasers, MRI scanners, nuclear energy and transistors, to name a few. Quantum mechanics helped us to understand and manipulate semiconductors, the key enabler for the transistor, meaning that computers, tablets and smartphones all exist because we know how to make use of the effects of quantum mechanics.
The kilogram
From May 2019, the definition of the kilogram is described by the laws of quantum mechanics.
Standards have undergone much-needed change throughout the last two centuries. Almost all standards in the International System of Units have now been redefined on the basis of physical constants rather than physical prototypes. The kilogram is the last unit of measure to be given a complete makeover. After almost 130 years, the definition of the kilo was changed.Up to May 2019, the unit was still based on a platinum-iridium alloy cylinder prototype created in 1889, which is kept in a vault in Paris and of which several replicas exist around the world.The new definition is based on the Planck constant, an important constant in quantum mechanics. This way, the definition of a kilogram is more accurate than basing it on a physical object. Also, there will no longer be special institutes that own the truth about the kilogram. It will be owned by everyone, described in a formula.