Battolyser Systems
Research Themes: Energy, chemistry, bio- & process technology

A TRL is a measure to indicate the matureness of a developing technology. When an innovative idea is discovered it is often not directly suitable for application. Usually such novel idea is subjected to further experimentation, testing and prototyping before it can be implemented. The image below shows how to read TRL’s to categorise the innovative ideas.
Summary of the project
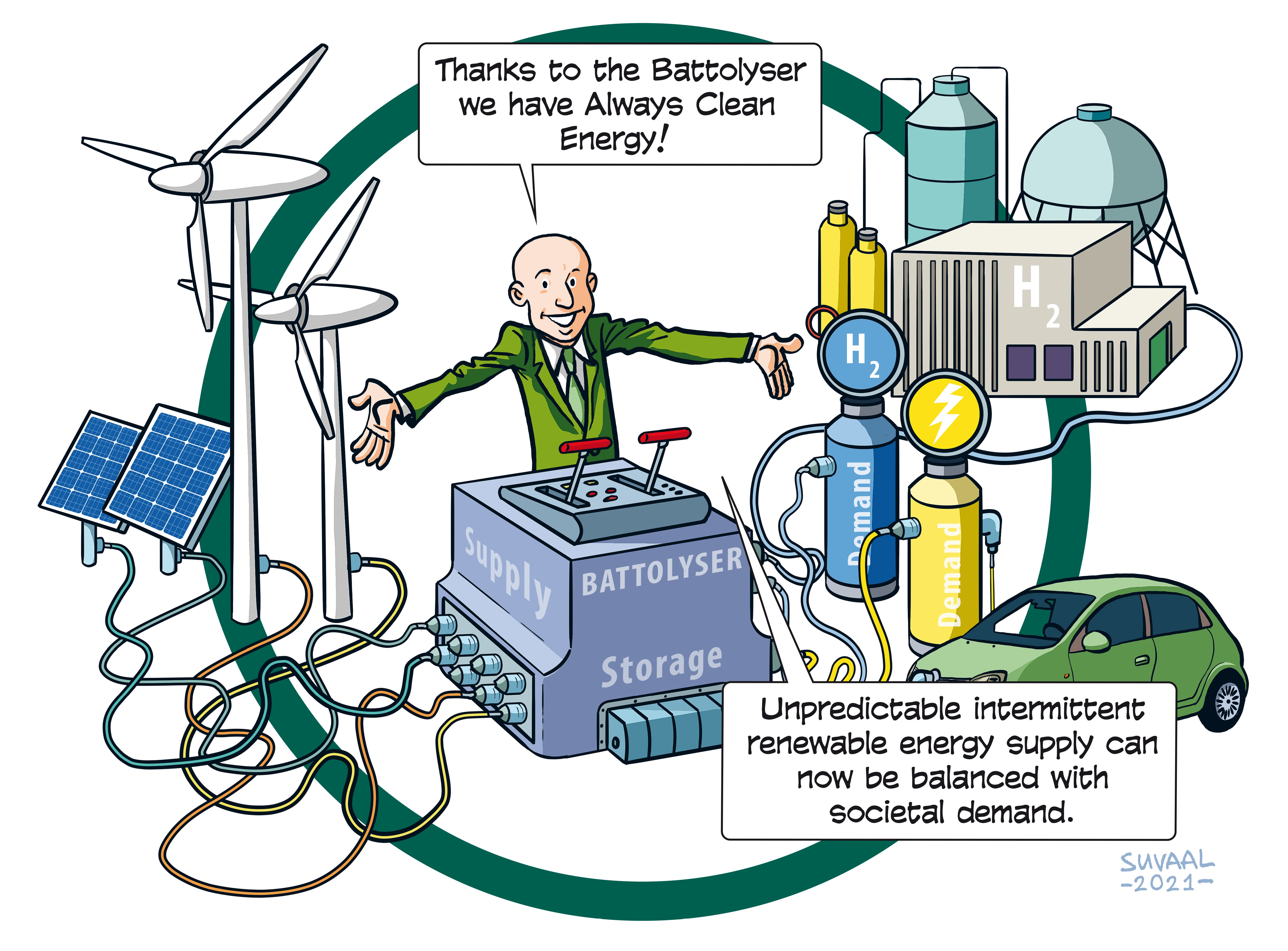
Currently, the intermittency of renewable energy generation makes it difficult to meet the society’s energy and feedstock needs. When the sun is shining, there is abundance of electricity, which makes it physically difficult and financially disadvantageous to sell overproduction to the grid. When there is a lack of renewable energy generation, we need new solutions to bring green energy back into the grid. Storing the surplus of electricity in the form of hydrogen can help balance these markets.
Batteries and electrolysers only offer partial solutions. Batteries can store surplus electricity, but have limited storage and aren’t well-suited for deep cycles in which they unload completely. Electrolysers can generate hydrogen from electricity, but cannot be switched off easily, meaning you have to run them for longer times, also when the electricity is too expensive. Integrating the two gives you a very special flexibility. You actually get a battery that can charge cheap electricity and when full continues as electrolyser producing low cost hydrogen, but that can switch as fast as a battery too selling electricity when it is expensive. Due to this arbitrage function, the Battolyser has a very high load factor.
The Battolyser provides a balancing power in the electricity market and converts surplus power into hydrogen. This creates a brilliant and unique business model for its clients, both big energy and chemical companies but also distributed, smaller parties in e.g. the distribution grid or agricultural sector.
Combining a battery and an electrolyser not only makes the Battolyser more flexible, but also more efficient and robust. The electrodes of the battery can be used to generate hydrogen when the battery is full. This means a huge surface area of electrodes is available for hydrogen production, which means that the current densities passing through those electrodes are very low, so that the resistance is very low and therefore the efficiency is very high. The charged electrode materials that are formed when charging the battery, are catalysts for the electrochemical reaction to produce hydrogen and oxygen. When the Battolyser discharges power to the grid, these charged layers of materials are converted to the discharged state again. In other words: the catalyst is regenerated and passivated again each cycle, creating a robust system in which catalysts cannot degrade.
What's next?
The Battolyser is currently experimenting with demonstrators (TRL 6-7), and is working on increasing its scale to provide Megawatt capacities. To do so, they currently grow their team for which they see much interest even in the growingly competitive market.
Contribution to the Energy transition?
A Battolyser balances the electricity grid by delivering energy at a high price to the grid when there is a shortage; and by cheaply buying energy from the grid for battery capacity charging and hydrogen production when there is a surplus.
