Unravelling the secrets of life with super-resolution microscopy
While the foundations of microscopy were laid more than three centuries ago by people like Antonie van Leeuwenhoek, the field has developed at lightning speed in the last decade. Dr. ir. Carlas Smith is now working on a new breakthrough: a microscope that combines various techniques to reveal what really happens inside living tissue.
Delft resident Antonie van Leeuwenhoek was the founder of microscopy, Carlas Smith's field. So it’s understandable that Smith still feels Van Leeuwenhoek's influence. “Although I did stints as a researcher in Boston and Oxford, as an academic I am rooted in Delft. When I studied physics here, I was already very interested in life science and technology. This was how I came across Van Leeuwenhoek's work.”
“There is another connection to Van Leeuwenhoek: via a quote by German scientist Stefan Hell, a Nobel laureate in my field. He stated that until the end of the nineteenth century, lens makers were the rulers of our field. With the best lenses you could attain the best resolution. First and foremost, Van Leeuwenhoek was one of these lens makers. Hell added that this is no longer the case in microscopy. It is now an interplay where opto-mechatronics is cleverly combined with algorithms.”
“Galileo is another source of inspiration for me. His motto, ‘measure what is measurable and make measurable what is not’, appeals to me. My fascination is observing things we've never observed before, in biology in particular. For example, during my PhD research in the US, we saw for the first time how an mRNA molecule moves from the nucleus of the cell to the rest of the cell; a highly essential biological process. This was extremely exciting.”

Carlas Smith
Super-resolution microscopy
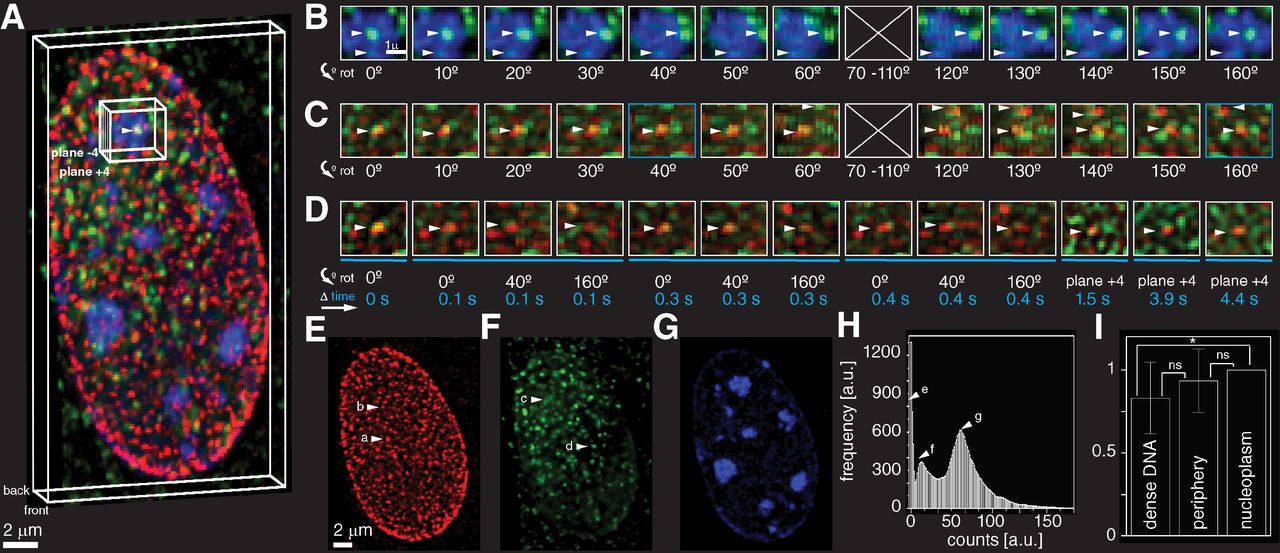
Super-resolution microscopy is the field Carlas Smith is involved in, particularly for the examination of biological tissues. “In physics, there are technologies such as the electron microscope, which offer a much higher resolution than is the case in current super-resolution microscopy. So why do we still need anything else? The difference is that super-resolution microscopy offers a much greater contrast and specificity because you use fluorescence. It is like pairing a beacon of light with the molecules you want to see, while around them is darkness. In addition to higher contrast and specificity, it is the ability to study living organisms why biologists find it such a valuable technology.”
“Super-resolution microscopy allows us to examine inside living cells. The technology uses the luminescent proteins originally found in jellyfish. Researchers can use gene editing to attach these fluorescent proteins to molecules. When such proteins are illuminated with a laser, they emit light shortly afterwards. Sensitive sensors record these light signals, and researchers can use algorithms to extract valuable data from the noise to construct images. While an ordinary optical microscope can create images at a scale of about a quarter micron, super-resolution microscopy can do so almost 100 times more accurately.”
My fascination is observing things we've never observed before, in biology in particular.
“The field of super-resolution microscopy has developed at lightning speed over the past decade. As early as 1995, a paper was published that stated, albeit rather abstractly, that if it is possible to distinguish molecules in a cell from each other, they can also be viewed, localised and tracked individually. The big breakthrough came in 2006. In the run-up to that essential publication, fluorescent molecules were discovered that would flash under certain conditions.
If everything was dark and only one molecule 'switched on', it could be distinguished. It was immediately understood that these molecules held great promise. This lead to the Nobel prize in 2014.”
“Subsequently, super-resolution microscopy developed rapidly, for example by expanding from 2D to 3D. Another important step was taken in 2017. By making clever use of the structure of the exposure, the resolution could be further improved. In 2019, we achieved significant improvements in Delft. By alternating and shifting horizontal and vertical light patterns across the sample, the locations of molecules can be determined very accurately. This Delft super-resolution innovation doubled the resolution.”
Correcting the Nobel laureate
Smith's research group made another notable scientific contribution last year. They published an important scientific correction: to the work of the aforementioned Nobel laureate Stefan Hell, no less. In 2020, researchers at Hell's lab suggested that through their Iterative Single-Molecule Localization Microscopy, resolution would improve much further. However, the researchers at TU Delft proved mathematically that in practice, these large resolution improvements are virtually unfeasible. In practical circumstances, the best possible outcome is an improvement of about five times compared with the standard technology. The field had generally assumed much greater potential. However, the scientists from Delft demonstrated, with a different mathematical approach, that the resolution improvements of Hell's group are difficult to achieve in practice.
The future of microscopy
“The field is now at a fork in the road, you might say. After all, the mystery of life is hidden deep inside the cell, smaller than a millionth of a metre, and happens at lightning speed, in under a tenth of a second. Some microscopes can zoom in on the smallest details, while others can take fast pictures, but there is no technology as yet that can do both. So you can either attain a very high resolution in space, or you can attain a high time resolution. And you certainly need the latter to be able to better track all manner of fast biological processes over time. The aim is to combine the two. We are quite unique, I think, in the sense that we are paying a lot of attention to this.”
“To make progress in this regard, I submitted a Vidi proposal, which was awarded in 2023. The result is recognition of our research, as well as further scaling up. We are now developing a microscope built from multiple microscopy techniques and building an algorithm to cleverly combine the images that are taken. We combine parallel data and 'merge' them. You have one measurement with a level of uncertainty and another with a level of uncertainty. If you combine the two, you end up with a smaller uncertainty. Although that insight in and of itself is not new, to realise it, you need to deal with all sorts of different technical challenges. For example, you need to build a new instrument that can handle different technologies, make cells that are suitable for this purpose and an algorithm that can handle it all.”
You could say that with this new microscope, we are creating the hammer and screwdriver of the future.
“This comes down to quite a set of engineering challenges. You have to work on every single aspect. The combination of optics, nanobiology, mechatronics and algorithms is essential. 'Delft' traditionally excels in optics and nanobiology, but here at the Centre for Systems and Control, part of the Faculty of Mechanical Engineering, we are very good at signal processing and mechatronics. So we have a very strategic position in Delft, which is not easily achieved. Traditionally, we used to wield the hammer and screwdriver here, as it were. You could say that with this new microscope, we are creating the hammer and screwdriver of the future.”