Breeding bacteria to build chemical compounds with electricity
Large quantities of useful chemicals and fuels, produced by bacteria that live on a diet of carbon dioxide and sustainable electricity. Though this might sound like a dream, it is a scenario that assistant professor Ludovic Jourdin aims to make come true within a few years.
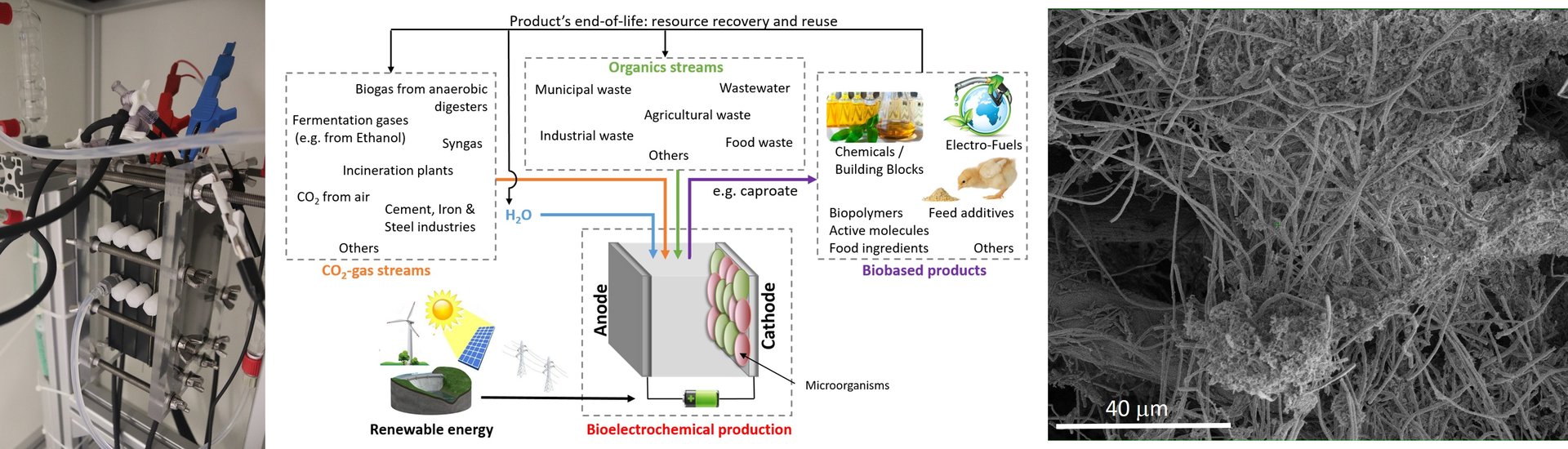
Our current chemical industry is primarily based on breaking down fossil resources into useful chemical building blocks. Not only are these fossil resources becoming scarce, but also the current production processes lead to high carbon dioxide emissions. At Delft University of Technology, a multidisciplinary team of over forty researchers is working on a so-called electrochemical alternative: they want to use renewable energy from for example solar panels and wind turbines, to fuel an electrochemical cell. Such a cell contains electrodes submerged in a conducting fluid, and dissociates molecules like carbon dioxide or nitrogen to synthesize the fragments into basic industrial feedstock such as methane, ethanol, carbon monoxide, carboxylic acids, or ammonia.
Ambitious goal
‘We have set ourselves an ambitious goal,’ Ludovic Jourdin explains. ‘In five years’ time, we want to have established a demonstrator refinery that is able to deliver 100 kilograms of product per day.’ To achieve this goal, the so called e-Refinery consortium is investigating three different routes, one of which is based on using living bacteria to perform the conversion of emission gases like carbon dioxide into chemical compounds. This is where Jourdin’s group Microbial Electrochemistry and Technology in the department of Biotechnology comes in. ‘In my research, I am investigating if we can use electrodes covered with a film of bacteria to break the chemical bonds between the carbon and the oxygen, and to transform the fragments into useful products.’
The first aim is to make molecules containing either two, four or six carbon atoms, like acetic, butyric, and hexanoic acids or their respective alcohols, with wide commercial application ranges. ‘Ultimately, we hope to be able to make an impact towards the production of molecules containing six carbon atoms, since they can be used as additives for the pharmaceutical industry, the cosmetics industry, the food and feed industry, or as precursor molecules for the production of plastics and fuels.’
Jourdin has an impressive track record in the young field of electricity-driven bioconversions research. ‘I currently hold the world record in the reactivity of the bacteria,’ he says with pride. He did obtain his record-breaking bacteria culture in a rather unusual fashion though, he laughingly admits. ‘I was working on my PhD research at the University of Queensland, and scooped up some bacteria-containing sludge from the bottom of a lake there. I mixed up these anaerobic bacteria, who can live without oxygen, in a “hit and miss” strategy to find an appropriate culture that could serve my purposes. By feeding them electricity only, I eventually managed to grow an enriched bacteria culture that was able to reconfigure carbon dioxide into acetate in an electrochemical cell.’ Ever since, Jourdin has taken his culture with him to continue his research, first at Wageningen University and Research and as of 2018 at Delft University of Technology.
His group is involved in both fundamental and applied research, going all the way from understanding the metabolism of the bacteria to reactor design and processing, he says. ‘Eventually, we want to develop a reactor design that overcomes limitations of the technique on all different scales. That is from the micrometer, so the scale of the organisms, to the scale of the reactor itself.’
Ample advantages
Although working with bacteria imposes some challenges, the technique has too many advantages to not look into it, Jourdin is convinced. ‘Current bacterial systems have slightly lower production rates, but much longer operation times. Moreover, bacteria are self-repairing and renew themselves.. Besides this, the bacteria we use are widely available, so they make up very cheap catalysts. Furthermore, where chemical catalysts usually can produce small molecules only, complex organisms like bacteria can produce a wide product spectrum with a higher end value. My current mixture, for example, is able to produce three different products, in different concentrations. And finally, since they are living organisms, bacteria are subject to evolution. So they might be able to adapt to changing circumstances, like changing compositions of the feedstock. That would be very beneficial if we would want to go to converting biomass or to capturing carbon dioxide directly from industrial flue gases.’ The idea of using bacteria for industrial scale processes is not new, Jourdin stresses: ‘There are already examples of large scale bacteria based plants which lead to consistent outcomes, for example in wastewater treatment. I am confident we can achieve the same in an electrochemical setup.’
To be able to improve the productivity of the system, Jourdin and his group first want to gain a fundamental understanding of the bacteria culture: Which bacteria are present and which of them are doing what? How can you control the outcome of the process? What happens for example when you change the temperature, the electricity supply, the pressure, or the purity of the carbon dioxide feedstock? ‘We want to use all of this knowhow to bring this idea to large scale applications. When you scale up the system, the dynamics change. We need to know how that influences the work of the bacteria. The crazy vision here is to learn from our current system to ultimately engineer dedicated microorganisms, the same way we did in fermentation.’
Brighter future
Jourdin is a strong believer of electrochemistry as the way forward for our modern day society. ‘We need to revolutionize chemical industry, and we need to use waste and renewable electricity to make this happen. We cannot afford to take thirty years to achieve this. That is what attracted me to take up this research position in Delft: the Department of Biotechnology and the e-Refinery’s ambitious, joint vision on the future of electrochemistry. The consortium brings all necessary expertise together to make this happen: from chemistry and material sciences, to microbiology, electrical engineering and policy management. It is great to be a part of such an innovation hub, where we develop something completely new and work on a brighter future for humankind.’