Activate high contrast
To main content
Stories of scientists
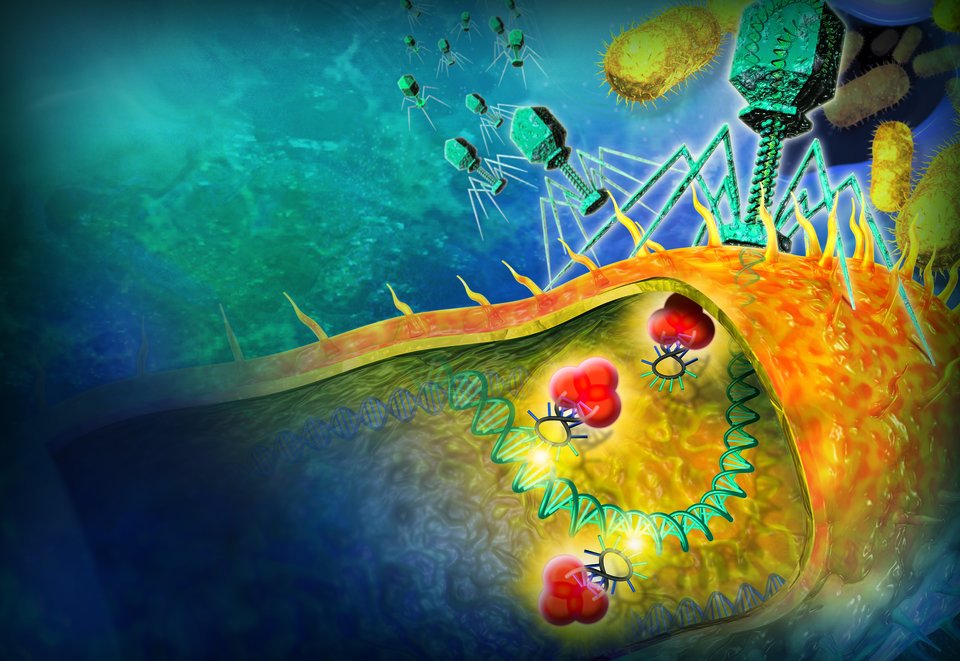
Bacteriophages as a possible alternative to antibiotics
What is invisible and will kill more people than cancer in thirty years' time? Answer: bacteria. Years of excessive use of antibiotics has enabled certain bacteria to evolve in a way that has become impossible to treat. Stan Brouns from TU Delft is conducting fundamental research into a possible alternative to antibiotics: bacteriophages. These days, researcher Stan Brouns’ mailbox is full of heart-wrenching stories about people who have contracted a superbug. Since he told about the possible use of bacteriophages to tackle antibiotic-resistant bacteria on Dokters van Morgen, patients with these bacteria in their system have been turning to the molecular microbiologist for help. ‘I just spoke to someone who was ringing about his daughter’, says Brouns. ‘She had a skin infection that wouldn't go away.’ Some of the patients have bad infections and are seriously ill. Antibiotics don’t help. As always, the million-dollar question is: can ‘these bacteriophages’ turn the tide? Balanced warfare What are bacteriophages? Quite simply, they are minuscule viruses that infect bacteria, which then use the bacteria cell as an organic factory to multiply themselves. They aren't particularly impressive: an angular head with a bit of DNA, a tubular neck and a couple of wispy legs. That is all they are. But despite their seemingly simple anatomy, bacteriophages are highly efficient killing machines. They kill a staggering third of all bacteria in our oceans. Not per year, not per month, but every single day. Bacteriophages and bacteria have been keeping each other in equilibrium for billions of years. Bacteria have developed an arsenal of defensive measures to fend off their arch enemies during the course of this ongoing war with bacteriophages. But evolution has equipped the phages with even better combat techniques to allow them to cross the bacteria’s defence lines. This biological warfare has enabled bacteriophages to become highly specialised. Most phages (as we call them) cannot infect more than one strain of bacteria. But – they are exceedingly good at it. Bacteria devourer The French-Canadian microbiologist Felix d’Herelle discovered bacteriophages 100 years ago. World War I had turned Europe into a battlefield, and in the midst of this chaos, d’Herelle was investigating an outbreak of dysentery among the soldiers in France. He made filtrates of faeces samples from a number of soldiers who had somehow managed not to succumb to the disease. He then mixed these bacteria- free filtrates with strains of Shigella bacteria, the pathogen that caused the dysentery outbreak. He cultivated the mixture to observe how the bacteria would grow under the influence of the bacteria-free faeces samples. When clear patches appeared in the mixture, d’Herelle instinctively understood what was happening: there was an invisible microbe in the bacteria-free mixtures, he wrote in his notes, which was attacking the bacteria. He thought that it was probably a virus that infiltrated bacteria. Not much later, the scientist gave the ‘bacteria viruses’ the name we still use today: bacteriophage, a compilation of ‘bacteria’ and the Greek word ‘phagein’, which means to ‘eat’ or ‘devour’. So bacteriophage literally means ‘bacteria devourer’. Medical legacy In the same way as he immediately realised what was happening in his Petri dishes, d’Herelle also understood the implications of his discovery. He experimented with the phages as a method for treating dysentery in the Hôpital des Enfants-Malades in Paris, and later developed several drugs based on bacteriophages. As it happened, antibiotics were discovered around the same time. These drugs also killed bacteria, and even more efficiently than phages. As an antibiotic works on a whole spectrum of bacteria rather than one specific strain, doctors did not need to know exactly which bacterium they were dealing with. Efforts were therefore focused on developing antibiotics. What happened to the phages? They slipped into oblivion. At least, they did in the West. But after World War II, doctors behind the iron curtain were finding it difficult to get hold of antibiotics. The Eastern Bloc was only too happy to inherit the Frenchman d’Herelle’s medical legacy. Phages became their most important weapon for fighting infections. Unlike in the West, in countries such as Georgia and Russia they continued developing phages as a medicine, and even set up so-called ‘phage banks’. Bacteriophages attack a bacterium. This image has been made by means of a transmission-elektron-microscope. Unfortunate immutability With the benefit of hindsight, we would clearly have done better to continue the work on phages. As demonstrated by the stories Stan Brouns receives in his mailbox every day, antibiotics are gradually losing their power. Increasingly more bacteria are adapting and making themselves immune to certain types of antibiotics. In fact, superbugs have evolved, with the ability to survive treatment with every type of antibiotic currently known to man. The number of patients dying from a simple infection is rising every year. One of the original advantages of antibiotics, their immutability, has become a serious disadvantage. So what will happen? It is estimated that by 2050, some 10 million people a year will die after being infected with antibiotic-resistant bacteria. The risk of infection from these superbugs will make routine operations life-threatening events in the ‘post-antibiotic era’. Science is doing all it can to develop new antibiotics, but the question is whether – and more importantly when – they will arrive. There is an increasing call for the West to pick up where d’Herelle left off, and start investigating the possibilities of bacteriophage-based drugs. Incorporating information The huge gaps in our knowledge mean that there is still a lot we don't know about phages. Stan Brouns’ research group at TU Delft is conducting fundamental research into the biology of bacteriophages and the way they interact with bacteria. One of the researchers in his group is examining the information exchange between various types of bacteriophages, for example. ‘Phages can incorporate information from other phages into their own genome, which allows them to infect a completely different type of bacterium’, says Brouns. ‘One of the aspects we are studying is the efficiency of this exchange principle.’ Another researcher in his group is trying to find out how bacteria can become resistant to phages. Something that is becoming increasingly obvious is that the minuscule bacteria-killers are much more complex than one would assume. ‘For example, they appear to work alongside our immune system when fighting infections’, explains Brouns. In recent tests, healthy mice and mice with a weakened immune system were infected with a bacterium. Phages were used to see whether they could beat the infection. Brouns: ‘The mice with a healthy immune system recovered, but the mice with a defective immune system did not. This suggests that the immune system and phages sometimes work together.’ Medical tourism So are phages really a wonder drug, the ultimate solution to our problems with antibiotic resistance? If only. ‘Not all problems relating to antibiotic resistance can be solved with phages’, says Brouns. In addition, we do not yet know how phages will affect the human body. Brouns: ‘We may very well develop antibodies against the viral particles, making them increasingly less effective.’ It is even possible that our immune system will attack certain phages, causing an allergic reaction or worse. Aspects like these need to be studied in more detail before bacteriophages can be introduced here as a legal drug. In addition, research must meet our high Western standards, demonstrating evidence of effectiveness and safety from clinical trials on groups of patients and control groups. The phage research conducted in Eastern Europe does not meet these standards. And then, of course, there is the pharmaceutical industry. Unfortunately, phages are tricky customers for a business case. After all, how can you apply for a patent for something that keeps mutating and changes every day? Also: if a drug is so effective that patients are better in no time, is it commercially viable? So Brouns has decided to roll up his own sleeves. ‘I am trying to get the go-ahead for a kind of phage bank here, to put phage therapy on the map.’ Contribution At present, the sad reality is that people in this country who contract an antibiotic-resistant bacterium are sometimes beyond help. If these people want to undergo phage treatment, they have to go abroad. The costs for medical tourists who see phages as a last resort run into thousands of euros. Brouns is convinced that phages may be a lifeline for patients who have already tried everything that regular medicine has to offer. So he uses his www.fagenbank.nl website to answer frequently asked questions about treatment with bacteriophages and advise people about medical trips to the Eliava Institute in Georgia. He hopes that his appearances in the media and initiatives like his website will set the ball rolling. According to Brouns, it would make a huge difference if Dutch healthcare insurance companies were to reimburse the cost of phage treatment abroad. ‘Insurers may be willing to pay for the treatment if they see that it works. Quite a lot of people have already been successfully treated in Georgia, so I am hopeful.’ Stan Brouns +31 15 27 83920 S.J.J.Brouns@tudelft.nl This is a story from Applied Sciences These days, researcher Stan Brouns’ mailbox is full of heart-wrenching stories about people who have contracted a superbug. Since he told about the possible use of bacteriophages to tackle antibiotic-resistant bacteria on Dokters van Morgen, patients with these bacteria in their system have been turning to the molecular microbiologist for help. ‘I just spoke to someone who was ringing about his daughter’, says Brouns. ‘She had a skin infection that wouldn't go away.’ Some of the patients have bad infections and are seriously ill. Antibiotics don’t help. As always, the million-dollar question is: can ‘these bacteriophages’ turn the tide? Balanced warfare What are bacteriophages? Quite simply, they are minuscule viruses that infect bacteria, which then use the bacteria cell as an organic factory to multiply themselves. They aren't particularly impressive: an angular head with a bit of DNA, a tubular neck and a couple of wispy legs. That is all they are. But despite their seemingly simple anatomy, bacteriophages are highly efficient killing machines. They kill a staggering third of all bacteria in our oceans. Not per year, not per month, but every single day. Bacteriophages and bacteria have been keeping each other in equilibrium for billions of years. Bacteria have developed an arsenal of defensive measures to fend off their arch enemies during the course of this ongoing war with bacteriophages. But evolution has equipped the phages with even better combat techniques to allow them to cross the bacteria’s defence lines. This biological warfare has enabled bacteriophages to become highly specialised. Most phages (as we call them) cannot infect more than one strain of bacteria. But – they are exceedingly good at it. Bacteria devourer The French-Canadian microbiologist Felix d’Herelle discovered bacteriophages 100 years ago. World War I had turned Europe into a battlefield, and in the midst of this chaos, d’Herelle was investigating an outbreak of dysentery among the soldiers in France. He made filtrates of faeces samples from a number of soldiers who had somehow managed not to succumb to the disease. He then mixed these bacteria- free filtrates with strains of Shigella bacteria, the pathogen that caused the dysentery outbreak. He cultivated the mixture to observe how the bacteria would grow under the influence of the bacteria-free faeces samples. When clear patches appeared in the mixture, d’Herelle instinctively understood what was happening: there was an invisible microbe in the bacteria-free mixtures, he wrote in his notes, which was attacking the bacteria. He thought that it was probably a virus that infiltrated bacteria. Not much later, the scientist gave the ‘bacteria viruses’ the name we still use today: bacteriophage, a compilation of ‘bacteria’ and the Greek word ‘phagein’, which means to ‘eat’ or ‘devour’. So bacteriophage literally means ‘bacteria devourer’. Medical legacy In the same way as he immediately realised what was happening in his Petri dishes, d’Herelle also understood the implications of his discovery. He experimented with the phages as a method for treating dysentery in the Hôpital des Enfants-Malades in Paris, and later developed several drugs based on bacteriophages. As it happened, antibiotics were discovered around the same time. These drugs also killed bacteria, and even more efficiently than phages. As an antibiotic works on a whole spectrum of bacteria rather than one specific strain, doctors did not need to know exactly which bacterium they were dealing with. Efforts were therefore focused on developing antibiotics. What happened to the phages? They slipped into oblivion. At least, they did in the West. But after World War II, doctors behind the iron curtain were finding it difficult to get hold of antibiotics. The Eastern Bloc was only too happy to inherit the Frenchman d’Herelle’s medical legacy. Phages became their most important weapon for fighting infections. Unlike in the West, in countries such as Georgia and Russia they continued developing phages as a medicine, and even set up so-called ‘phage banks’. Bacteriophages attack a bacterium. This image has been made by means of a transmission-elektron-microscope. Unfortunate immutability With the benefit of hindsight, we would clearly have done better to continue the work on phages. As demonstrated by the stories Stan Brouns receives in his mailbox every day, antibiotics are gradually losing their power. Increasingly more bacteria are adapting and making themselves immune to certain types of antibiotics. In fact, superbugs have evolved, with the ability to survive treatment with every type of antibiotic currently known to man. The number of patients dying from a simple infection is rising every year. One of the original advantages of antibiotics, their immutability, has become a serious disadvantage. So what will happen? It is estimated that by 2050, some 10 million people a year will die after being infected with antibiotic-resistant bacteria. The risk of infection from these superbugs will make routine operations life-threatening events in the ‘post-antibiotic era’. Science is doing all it can to develop new antibiotics, but the question is whether – and more importantly when – they will arrive. There is an increasing call for the West to pick up where d’Herelle left off, and start investigating the possibilities of bacteriophage-based drugs. Incorporating information The huge gaps in our knowledge mean that there is still a lot we don't know about phages. Stan Brouns’ research group at TU Delft is conducting fundamental research into the biology of bacteriophages and the way they interact with bacteria. One of the researchers in his group is examining the information exchange between various types of bacteriophages, for example. ‘Phages can incorporate information from other phages into their own genome, which allows them to infect a completely different type of bacterium’, says Brouns. ‘One of the aspects we are studying is the efficiency of this exchange principle.’ Another researcher in his group is trying to find out how bacteria can become resistant to phages. Something that is becoming increasingly obvious is that the minuscule bacteria-killers are much more complex than one would assume. ‘For example, they appear to work alongside our immune system when fighting infections’, explains Brouns. In recent tests, healthy mice and mice with a weakened immune system were infected with a bacterium. Phages were used to see whether they could beat the infection. Brouns: ‘The mice with a healthy immune system recovered, but the mice with a defective immune system did not. This suggests that the immune system and phages sometimes work together.’ Medical tourism So are phages really a wonder drug, the ultimate solution to our problems with antibiotic resistance? If only. ‘Not all problems relating to antibiotic resistance can be solved with phages’, says Brouns. In addition, we do not yet know how phages will affect the human body. Brouns: ‘We may very well develop antibodies against the viral particles, making them increasingly less effective.’ It is even possible that our immune system will attack certain phages, causing an allergic reaction or worse. Aspects like these need to be studied in more detail before bacteriophages can be introduced here as a legal drug. In addition, research must meet our high Western standards, demonstrating evidence of effectiveness and safety from clinical trials on groups of patients and control groups. The phage research conducted in Eastern Europe does not meet these standards. And then, of course, there is the pharmaceutical industry. Unfortunately, phages are tricky customers for a business case. After all, how can you apply for a patent for something that keeps mutating and changes every day? Also: if a drug is so effective that patients are better in no time, is it commercially viable? So Brouns has decided to roll up his own sleeves. ‘I am trying to get the go-ahead for a kind of phage bank here, to put phage therapy on the map.’ Contribution At present, the sad reality is that people in this country who contract an antibiotic-resistant bacterium are sometimes beyond help. If these people want to undergo phage treatment, they have to go abroad. The costs for medical tourists who see phages as a last resort run into thousands of euros. Brouns is convinced that phages may be a lifeline for patients who have already tried everything that regular medicine has to offer. So he uses his www.fagenbank.nl website to answer frequently asked questions about treatment with bacteriophages and advise people about medical trips to the Eliava Institute in Georgia. He hopes that his appearances in the media and initiatives like his website will set the ball rolling. According to Brouns, it would make a huge difference if Dutch healthcare insurance companies were to reimburse the cost of phage treatment abroad. ‘Insurers may be willing to pay for the treatment if they see that it works. Quite a lot of people have already been successfully treated in Georgia, so I am hopeful.’ Stan Brouns +31 15 27 83920 S.J.J.Brouns@tudelft.nl This is a story from Applied Sciences Related stories Affordable MRI Constructing living cells Tinkering under the bonnet of life
In search of better Li-ion batteries and alternatives
There is a lot of progress still to be made when it comes to batteries and energy storage. The problem of storing energy is often underestimated however. Gradual evolution in the world of batteries is more likely than a revolution. TU Delft's batteries lab will focus primarily on research into potential alternatives for what is currently the standard technology: the lithium-ion battery. Confusing times "We are living in confusing times when it comes to batteries and energy storage", says TU Delft's professor Fokko Mulder. In his view, misconceptions have emerged about what is and is not possible in terms of energy storage, partly because of the rise of renewable sources such as wind and solar energy. (The misconceptions start around the fact that only about 13% of the energy we consume ‘consists of’ electricity, but that is likely to be significantly higher in a renewable future). Large-scale energy storage is essential because the supply of power from renewable energy sources, such as wind and solar energy, fluctuates by its very nature. You cannot store electricity, which means alternative solutions need to be devised. "Current expectations are slightly too high with regard to the rapid introduction of renewable energy and the potential role of batteries in large-scale energy storage. For example, a lot can be achieved in the field of energy storage and buffering using batteries in electric cars. But that method can only be used to store a very small portion of future renewable electricity production, even if everyone in the Netherlands were to drive fully electric vehicles." "Technologically and financially, it comes down to the fact that batteries alone are simply not sufficient to offset all of the major peaks and troughs in renewable energy production across the year", Mulder explains. "Storage over a longer period is particularly inefficient and expensive using batteries. Completely different systems are required for that." Whatever the case, there is a real need for electricity storage, both for the short and longer terms (weeks or seasons). Although batteries are best for short-term storage, artificially-produced fuels such as hydrogen are most suitable for long-term energy storage. Battolyser Mulder sees more potential in an alternative, combined approach for the large-scale storage of energy from renewable sources. The ‘battolyser’ developed by his group is an example of this. It is based on an old-fashioned nickel-iron battery. During charging, the electrodes on this battery form two materials (NiOOH and reduced Fe). In the world of electrolysis, these materials are known as the catalyst for the electrochemical reaction that produces hydrogen and oxygen. In their charged state, the electrodes enable water to be electrolysed. According to Mulder, the fact that nickel-iron batteries also produce hydrogen gas while charging was always regarded as a drawback and that was one of the reasons why other types of battery were ultimately more successful. Now, though, this could actually turn out to be an advantage. "Electricity and hydrogen have always been seen as two separate, even competing, solutions for energy storage," says Mulder. "With the battolyser we have the first integrated battery electrolysis system, which can store and supply electricity very efficiently as a battery, and when the battery is full, it automatically starts splitting water into hydrogen and oxygen using electrolysis. The battolyser has also been found to be stable, both in battery and electrolysis mode, even under long, intensive charging, discharging and hydrogen production. This means that the battolyser brings together the infrastructure for electricity storage with that for hydrogen production in a natural way for the very first time. We need both battery storage and fuels." Safety All things considered, Mulder expects to see gradual progress in the development of energy storage and batteries, driven by an increasing need for storage. Dr Erik Kelder from TU Delft's Fundamental Aspects of Materials and Energy (FAME) group confirms this view. "In the future, I think it is much more likely that we will see evolution in the world of batteries rather than a revolution." According to Kelder, there has been significant growth in battery research in recent years and it has gained in strategic importance. Mobile applications are the main driver for batteries. But batteries have also become important tools for applications other than mobile electronics, such as electric vehicles and human implants. Viewed strategically, Europe has only a very small portion of world production. China, South Korea, Japan and the US are clearly more important players. "There are three determining factors in the development of batteries: safety, cost price and energy density (the amount of energy you can store in a battery). Ultimately, everything in the battery world starts with safety. Just one incident can leave you right back where you started", says Kelder. "Aside from safety, the main focus of science is on increasing energy density, enabling more energy to be stored in a battery." Currently, the standard technology is the lithium-ion battery. Kelder believes that this still has some potential for technological development. "However, the best you can probably hope for is an increase in energy density by a factor of 1.5. If you want to make major progress in energy density, there needs to be an alternative for the Li-ion battery." Strategic Developing alternatives was precisely the reason why the new TU Delft batteries lab was launched earlier this year (officially opened in April at the Reactor Institute Delft or RID). Teams of researchers are working there on the next generation of batteries. Kelder explains that, from a scientific perspective, there are currently numerous options on the table. "All kinds of different concepts are being researched. Personally, I think that a Li-sulphur variant could serve as an interim solution on the way towards a completely new generation of batteries." An alternative approach, such as replacing the lithium with sodium, would not provide much benefit, according to Kelder. "You would be better advised to replace the lithium with calcium or magnesium, for example. There are also other potential variants using zinc, air and water." Kelder points out that it is difficult to predict what the best option will be. But it is important to realise that these potential alternatives will probably need time before they are ready for use in applications and start to constitute a genuine threat to Li-ion batteries. "That could actually take decades. Above all, there needs to be a lot more fundamental material research." Visible So it was clearly high time for a new batteries lab. This one can even claim to be the only one of its kind in the Netherlands. It has been enlarged and offers even more possibilities for research. In addition, all facilities in the labs are centrally located alongside each other and can be combined with the infrastructure of the RID, including ‘operando XRD’ techniques and neutron depth profiling, neutron diffraction and solid state NMR. These techniques can, for example, be used to make lithium atoms visible. In the lab, various coating and preparation techniques are applied and scanning electrochemical microscopy is linked with atomic force microscopy, enabling batteries to be viewed at atomic scale. This will make it possible to gain a better understanding of the complex processes in batteries, ultimately enabling better batteries to be developed. Fokko Mulder +31 15 27 85037 F.M.Mulder@tudelft.nl This is a story from Applied Sciences Confusing times "We are living in confusing times when it comes to batteries and energy storage", says TU Delft's professor Fokko Mulder. In his view, misconceptions have emerged about what is and is not possible in terms of energy storage, partly because of the rise of renewable sources such as wind and solar energy. (The misconceptions start around the fact that only about 13% of the energy we consume ‘consists of’ electricity, but that is likely to be significantly higher in a renewable future). Large-scale energy storage is essential because the supply of power from renewable energy sources, such as wind and solar energy, fluctuates by its very nature. You cannot store electricity, which means alternative solutions need to be devised. "Current expectations are slightly too high with regard to the rapid introduction of renewable energy and the potential role of batteries in large-scale energy storage. For example, a lot can be achieved in the field of energy storage and buffering using batteries in electric cars. But that method can only be used to store a very small portion of future renewable electricity production, even if everyone in the Netherlands were to drive fully electric vehicles." "Technologically and financially, it comes down to the fact that batteries alone are simply not sufficient to offset all of the major peaks and troughs in renewable energy production across the year", Mulder explains. "Storage over a longer period is particularly inefficient and expensive using batteries. Completely different systems are required for that." Whatever the case, there is a real need for electricity storage, both for the short and longer terms (weeks or seasons). Although batteries are best for short-term storage, artificially-produced fuels such as hydrogen are most suitable for long-term energy storage. Battolyser Mulder sees more potential in an alternative, combined approach for the large-scale storage of energy from renewable sources. The ‘battolyser’ developed by his group is an example of this. It is based on an old-fashioned nickel-iron battery. During charging, the electrodes on this battery form two materials (NiOOH and reduced Fe). In the world of electrolysis, these materials are known as the catalyst for the electrochemical reaction that produces hydrogen and oxygen. In their charged state, the electrodes enable water to be electrolysed. According to Mulder, the fact that nickel-iron batteries also produce hydrogen gas while charging was always regarded as a drawback and that was one of the reasons why other types of battery were ultimately more successful. Now, though, this could actually turn out to be an advantage. "Electricity and hydrogen have always been seen as two separate, even competing, solutions for energy storage," says Mulder. "With the battolyser we have the first integrated battery electrolysis system, which can store and supply electricity very efficiently as a battery, and when the battery is full, it automatically starts splitting water into hydrogen and oxygen using electrolysis. The battolyser has also been found to be stable, both in battery and electrolysis mode, even under long, intensive charging, discharging and hydrogen production. This means that the battolyser brings together the infrastructure for electricity storage with that for hydrogen production in a natural way for the very first time. We need both battery storage and fuels." Safety All things considered, Mulder expects to see gradual progress in the development of energy storage and batteries, driven by an increasing need for storage. Dr Erik Kelder from TU Delft's Fundamental Aspects of Materials and Energy (FAME) group confirms this view. "In the future, I think it is much more likely that we will see evolution in the world of batteries rather than a revolution." According to Kelder, there has been significant growth in battery research in recent years and it has gained in strategic importance. Mobile applications are the main driver for batteries. But batteries have also become important tools for applications other than mobile electronics, such as electric vehicles and human implants. Viewed strategically, Europe has only a very small portion of world production. China, South Korea, Japan and the US are clearly more important players. "There are three determining factors in the development of batteries: safety, cost price and energy density (the amount of energy you can store in a battery). Ultimately, everything in the battery world starts with safety. Just one incident can leave you right back where you started", says Kelder. "Aside from safety, the main focus of science is on increasing energy density, enabling more energy to be stored in a battery." Currently, the standard technology is the lithium-ion battery. Kelder believes that this still has some potential for technological development. "However, the best you can probably hope for is an increase in energy density by a factor of 1.5. If you want to make major progress in energy density, there needs to be an alternative for the Li-ion battery." Strategic Developing alternatives was precisely the reason why the new TU Delft batteries lab was launched earlier this year (officially opened in April at the Reactor Institute Delft or RID). Teams of researchers are working there on the next generation of batteries. Kelder explains that, from a scientific perspective, there are currently numerous options on the table. "All kinds of different concepts are being researched. Personally, I think that a Li-sulphur variant could serve as an interim solution on the way towards a completely new generation of batteries." An alternative approach, such as replacing the lithium with sodium, would not provide much benefit, according to Kelder. "You would be better advised to replace the lithium with calcium or magnesium, for example. There are also other potential variants using zinc, air and water." Kelder points out that it is difficult to predict what the best option will be. But it is important to realise that these potential alternatives will probably need time before they are ready for use in applications and start to constitute a genuine threat to Li-ion batteries. "That could actually take decades. Above all, there needs to be a lot more fundamental material research." Visible So it was clearly high time for a new batteries lab. This one can even claim to be the only one of its kind in the Netherlands. It has been enlarged and offers even more possibilities for research. In addition, all facilities in the labs are centrally located alongside each other and can be combined with the infrastructure of the RID, including ‘operando XRD’ techniques and neutron depth profiling, neutron diffraction and solid state NMR. These techniques can, for example, be used to make lithium atoms visible. In the lab, various coating and preparation techniques are applied and scanning electrochemical microscopy is linked with atomic force microscopy, enabling batteries to be viewed at atomic scale. This will make it possible to gain a better understanding of the complex processes in batteries, ultimately enabling better batteries to be developed. Fokko Mulder +31 15 27 85037 F.M.Mulder@tudelft.nl This is a story from Applied Sciences Related stories Modern trombe wall saves energie Summoning heat from below Intelligent, self-driving wind turbines
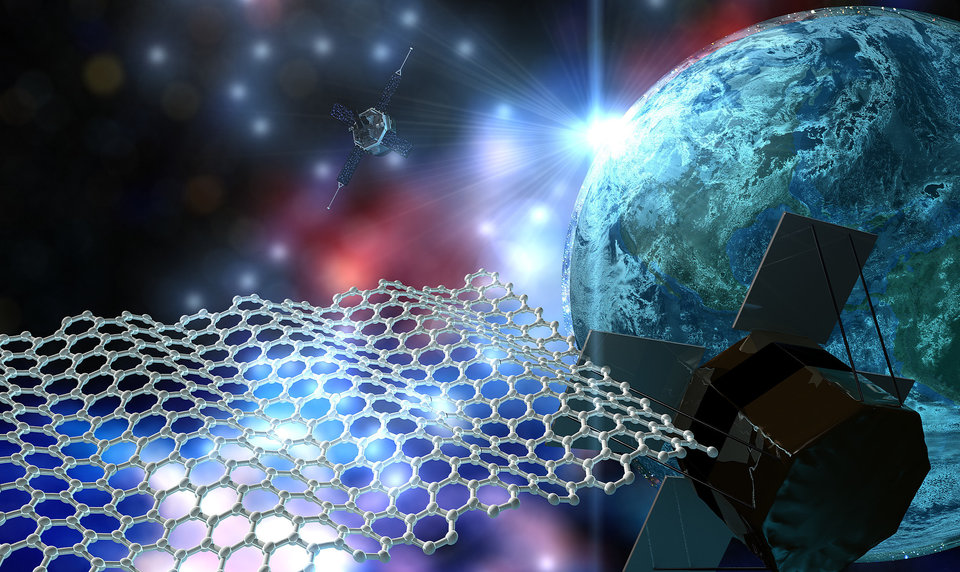
TU Delft tests graphene as a material for solar sails
Item 1 of 1 A team of researchers are preparing an exciting experiment to test graphene in space-like zero-gravity, for potential future use as a light sail in space-craft propulsion. The GrapheneX team successfully submitted their experiment proposal to ESA Education’s Drop Your Thesis! Programme in October 2016. The team members – Santiago Cartamil-Bueno, Rocco Gaudenzi, Davide Stefani and Vera Janssen – will travel to Bremen, Germany between 6-17 November 2017 to perform their experiment at the 146 m ZARM Drop Tower . Propelling without fuel Light sails can be used in space as a method of propelling spacecraft using light from the sun or from Earth-based lasers. When light is reflected from or absorbed by a surface, it exerts a force that pushes the surface away from the light source. This radiation pressure can be used to propel objects in space without using fuel or gases. However, the thrust generated by radiation pressure is very low. For effective propulsion, the light sail must have a large surface and be as light as possible. Graphene is very light and strong, and could be a good candidate for solar sails. The GrapheneX team plan to investigate how graphene could work as a light sail in an experiment that simulates the low-gravity and high-vacuum conditions of space. The sails to be tested by the GrapheneX team are graphene membranes, supplied by Graphene Flagship partner Graphenea. The radiation pressure from shining high-power laser light onto the graphene membranes will should cause the sails to move approximately 2 mm. This displacement will be measured with a simple microscope to determine the thrust on the graphene sails. The team plan to use different colours of laser light, to investigate the exact mechanism of how momentum is transferred to the graphene from light. Rise and fall At the ZARM Drop Tower, the experiment – including graphene sails, lasers and cameras - will be loaded into a capsule and catapulted the height of the tower. Inside the tower, vacuum conditions allow the capsule to rise and fall without friction or air resistance, so that the capsule experiences apparent weightlessness down to one-millionth of the Earth’s gravitational force. As the capsule accelerates, the graphene sails will be released into almost gravity-free free-fall. At this point, the laser will be allowed to shine onto the graphene chip, and the pressure generated by the laser falling onto the graphene will be detected. A key challenge of this experiment is automating the experiment procedures to initiate and record the results in the 9.3 seconds of free fall. All preparatory work for this experiment is being carried out by the GrapheneX team at TU Delft, with support from Herre van der Zant (TU Deflt), Angelo Cervone (TU Delft) and Jian Rong Gao (Delft Space Institute, the Netherlands). As part of the Drop Your Thesis! programme, the team are also mentored by Pekka Janhunen (Finnish Meteorological Institute, Finland). This experiment is supported by ESA Education, the Graphene Flagship and the Delft Space Institute. Follow the action on the blog! GrapheneX Twitter GrapheneX Facebook Zero Gravity Graphene - Solar Sails

Introducing... Kobus Kuipers
Professor Kobus Kuipers is Director of the department of Quantum Nanoscience (QN). Last November, he made the move to TU Delft from AMOLF, where he was group leader of NanoOptics and Head of the Center for Nanophotonics. How has he experienced the first 6 months of his Directorship? And what does he have planned for the department? Do you have any hobbies? ‘I absolutely love good food. I used to be a typical Saturday chef: I would plunder all the markets for the right ingredients and cook up something special with what I found. Nowadays, I no longer always have time to indulge my foodie side. Apart from that, I used to play table tennis and I still enjoy playing strategic games online.’ Light seems to be a theme running through your research. Is this true? ‘Yes, I call myself a “light person”. Put simply, I use nanostructures to do fun and interesting things with light. I am not one to blow my own trumpet, but I created the biggest stir with my slow light measurements.’ Slow light? Isn’t the speed of light constant? ‘No. You can pass light through a structure in which you periodically alternate different materials. I typically use a thin slice of silicon, into which I position a periodical grid of holes, approximately half a wavelength apart. If light wants to move within this construction, it needs to conform to the periodicity that has been introduced. That means that light behaves like electrons in an atomic crystal, and slows down or even stops for certain colours and/or places. You can therefore control the interaction between light and material. Incidentally, that is one of the reasons behind my decision to come to Delft. There are several material systems here in Delft that make me think: if we combine my expertise with the expertise already here, we should be able to look forward to some very exciting results.’ So the move was on the cards? ‘Every so often in the last few years, I was asked whether I wanted to leave AMOLF. AMOLF is a fantastic place and I really enjoyed being there, so my answer was usually an unhesitating “no”. But in 2015, I got a call from Tim van der Hagen, who was still Dean of the Faculty of Applied Sciences at the time. I realised that it was perhaps time for a change, and got talking with him. That is how I moved from one fantastic place to another, with the aim of broadening my horizons and taking my research to the next level.’ And has collaboration already flourished? ‘The academic interactions with the other PIs (Principal Investigators, ed.) are going well, and the first simple measurements are being prepared. Initial plans are now being laid for more in-depth collaboration.’ What is the culture like here, compared to at AMOLF? ‘It is different. For example, I get the idea that people here are quite focused on the United States. AMOLF also had an international focus, but it was a touch more ‘European’, I think. Researchers at AMOLF were also involved in education, but they do not teach as much as researchers here. That naturally takes up a chunk of their time. Having said that, other things are comparable. Apart from being tremendously intelligent and creative, people here are – to name just one characteristic – extremely dedicated. They are keen to succeed on the boundaries of what is possible, and to stand out while doing so. Everyone here is striving to be amongst the best in the world, as was also the case at AMOLF.’ And when it comes to the organisation? ‘That is also different, because at Delft, you are part of a (much) larger structure. That is reflected, for example, in the fact that some things take a little longer; the dynamics of the organisation are slightly different. I have an idea that the danger of individualism therefore looms more evidently here than at AMOLF.’ Will that be one of your focuses moving forward? ‘Yes, I would like to increase the ‘we feeling’. I think that there are gains to be made with respect to our sense of unity. We recently made a start – we had the first away-day since I came to Delft.’ Just to increase the ‘we feeling'? ‘Absolutely not, it naturally also has substantive benefits. During the away-day, I focused on three questions: ‘What are we good at?’, ‘What inspires us?’ and ‘What connects us?’. I asked my colleagues to brainstorm on these questions in small, alternating groups. It is now up to me to extract one or more themes from their answers that can help the abundant talent and expertise available at the university to live up to its promise. These themes will then provide a point of departure for follow-up sessions, which should lead to improved internal collaboration and, ideally, a new ‘Man on the Moon’ project. I believe that it is important that we become more than the sum of our parts.’ Any other news from the department? ‘Last year, the section structure at QN was replaced with a PI structure. A positive development if you ask me, but the advantage of the sections was that they provided an element of coherence and structure. Now, we are ‘flat’. We need to work together to ensure that this structure does not lead to disintegration. I have to admit that I actually enjoy thinking about how we can safeguard the coherence and how we can run the department efficiently.’ Did you not expect to enjoy that side of the job? ‘Well, I think that Tim primarily brought me on board as Departmental Director, and I see that role as a fine challenge. However, I made the move mainly because of my deep interest in the scientific side of things. When my lab was delayed, and I was here a month earlier than my group, it suddenly dawned on me how much I enjoyed pondering how to improve departmental processes.’

Bio-energy: not a bad idea
Biofuels don't have a particularly good reputation. Undeservedly so, says Professor Patricia Osseweijer from the department of Biotechnology. Bearing the United Nations Millennium Development Goals in mind, she is currently arguing the case for more investment in bio-energy. Not only because biofuels are renewable, but also because smart investment in bio-energy can play a part in the social development of deprived areas. Why do we need bio-energy when we've already got the sun and the wind as sustainable energy sources? ‘You have a point, but solar energy and wind energy are nowhere near enough to counteract climate change. Also, they’re not the best option for every place on Earth. One of the advantages of biofuels is that you need land to produce it. If you invest in biofuels, you don't only generate energy, but you also improve agriculture and create employment and income for people in poorer circumstances.’ Are there any success stories relating to biofuels? ‘Forty years ago, Brazil had political reasons for trying to become self-supporting in energy. The Brazilians invested heavily in growing sugar cane. Now, the sugar they produce is sold on the market, but if the price of sugar drops they can ferment it and convert it into alcohol. The end product is ethanol, and as ‘flex-fuel’ cars are now common in Brazil, Brazilians can fill their tanks with it. Cane sugar is grown on 1.4 percent of Brazil's agricultural land, which is a fairly small area. But it still produces around 50 percent of their total fuel requirement.’ Are we talking about half the fuel requirement for all vehicles? No, the 50 percent relates to private cars, in which ethanol replaces petrol. Freight traffic runs on diesel and the aviation industry relies on kerosene. You can't replace these with ethanol, but you could use biodiesel and biokerosene instead. We can already make these from used cooking fat, but technically it should also be possible to make bio-jet fuel from ethanol.’ What are the objections to producing energy from biomass? ‘There are two main objections. The first is that people think that there isn't enough land to feed a growing global population and grow crops for producing biofuels. ‘No food in fuel tanks’, as Oxfam Novab puts it. The second argument that is often put forward is that biofuels aren't actually as sustainable as they seem to be, because of the CO2 emissions when they are produced and used.’ Let's take a look at that second point. How sustainable is bio-energy compared with fossil fuels? ‘Oil has been in the ground for millions of years. When you burn it, the CO2 stored within is suddenly released into the atmosphere. Plants also store CO2 from the atmosphere so when you burn them, they also release CO2. But this cycle only takes a year at the most, so it's much shorter than the fossil fuel cycle.’ What about the argument that bio-energy is competing with food production? Is there any truth in that? ‘None whatsoever. Various studies, including some that we've worked on, show that there is enough land to grow crops for both food production and biofuels. Particularly in poorer areas.’ What sort of research is being carried out into biofuels in the department of Biotechnology? ‘To give a concrete example: Jack Pronk's group has developed a yeast that converts C5 sugars into ethanol. These C5 sugars used to be a residual product as yeasts could only cope with C6 sugars. The fact that we can now also convert these C5 sugars means greater yields. We’ve also got the Bioprocess technology group, which has computed huge amounts of data to find the most efficient way of setting up a production system. Yet another group is involved in enzymology, exploring which enzymes can be used to release residual matter from plants. This is essential because to get to the sugars, you first have to break down the cell walls. So our research groups complement each other nicely. And we also work closely with industry.’ Can you give some examples of partnerships? 'Certainly. We just launched a project with several companies that use plants (and residual plant matter) to produce ethanol, in which POET-DSM is taking part. A few years ago, they opened a biorefinery in the USA that derives ethanol from plant waste. This refinery, Liberty, uses the yeast developed here that I was just telling you about. We’re also working with SkyNRG, a spin-off of KLM that trades in bio-jet fuel, to look for better ways of organising the entire infrastructural system for producing biofuels so that farmers, for example, can become involved. In addition, Mark van Loosdrecht's group is studying residues from waste water to see how this could be used to make different products.’ In short: the circular economy? ‘Exactly. It's all about sustainability and the circular economy. We think that biofuels could play an important part in this, particularly in terms of social development in deprived areas. Ultimately, we want everyone in the chain to reap the benefits.’