Novel solid-state electrolytes
Novel Solid-State Electrolytes for Hydrogen
Purpose:
Ion conducting materials are important for a variety of purposes including batteries, electrolysers, electroconversion, and more. An obvious example is the Li-ion battery where the electrolyte is capable of transporting Li+ between the cathode and anode. Nearly all research roadmaps for Li-based energy storage point to the eventual use of solid-state electrolytes due to their enhanced safety and compact size compared to liquid electrolytes. In the meantime, research has also been done to find materials which can conduct other ions, for example Na+, Mg2+, Al3+, H+, O2-, F-, and others.
This project focuses on the discovery and understanding of hydride (H-) conducting solid-state materials. This could play a role in batteries, low-temperature alkaline electrolysis, and hydrogen fuel cells. In this way, this project aligns with our group’s other devices which aim to promote hydrogen as an energy vector and, more generally, devices for electrochemical conversion for sustainable fuels and feedstock.
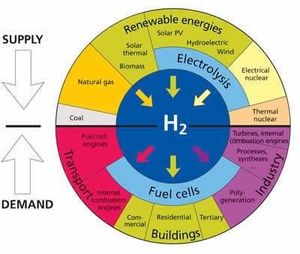
An explanation of proposed hydrogen economy where hydrogen can be supplied by the methods in the upper semicircle, and can be supplied to the devices in the lower semicircle to produce electricity and other products. Image adapted from Rebouillat, S. et. al., Int. J. Electrochem. Sci. 6, 5830-5917 (2011).
Aim:
It has been shown by a group in Japan (Institute for Molecular Science) led by G. Kobayashi that “oxyhydride” materials are capable of transporting negatively charged hydrogen ions (H-) without unwanted electronic conduction. Although a number of other oxyhydride compounds exist in the literature, only La2-x-ySrx+yLiH1-x+yO3-y has been proven to be a pure H- conductor.
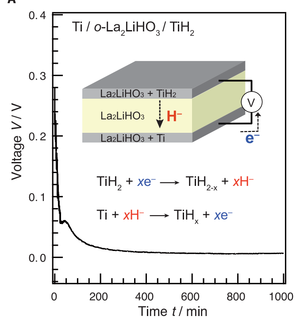
First concept of a device utilising an oxyhydride as the solid-state electrolyte. The electrodes consist of Ti and TiH2 whereby La2LiHO3 transports H- ions between them. Image adapted from Kobayashi, G. et al., Science 351, 1314-1317, (2016).
Our goal is to understand the mechanism of H- conduction as well as the material aspects necessary to facilitate this behaviour. Such an understanding can lead to the discovery of other materials capable of this ion mobility. Furthermore, this can help to improve the efficiency of mobility and maximise performance.
Approach:
The efficacy of yttrium oxyhydride as a solid-state H- conductor will be investigated first, as this material is well understood by our group. Perovskite and Ruddlesden-Popper oxyhydrides will be investigated as well. In many of these compounds, the ratio of hydrogen to oxygen can be varied by the reaction conditions. The goal is to make a correlation between the ionic conductivity and material properties such as hydrogen content, microstructure, lattice parameters, bandgaps, electron densities, vacancy structures, local hydrogen environments, etc..
Thin-films of these materials will be made by reactive magnetron sputtering and pulsed laser deposition. Analysis will be carried out by electrochemical impedance spectroscopy, NMR, positron annihilation spectroscopy, muon spin rotation, XRD, optical transmission, and others.
Collaboration:
- Arno Kentgens (Radboud Universiteit Nijmegen, Netherlands)
- Gilles de Wijs (Radboud Universiteit Nijmegen, Netherlands)
- Shrestha Banerjee (Radboud Universiteit Nijmegen, Netherlands)
- Marnix Wagemaker (Reactor Institute Delft, Netherlands)
- Frans Ooms (Reactor Institute Delft, Netherlands)
- Thomas Prokscha (Paul Scherrer Institut SμS, Switzerland)
Contact:
Bernard Dam (b.dam@tudelft.nl) en Stephan Eijt (s.w.h.eijt@tudelft.nl)