Optical hydrogen sensors/detectors / Hydrogenography of metal hydrides
With the increasing number of hydrogen applications the need for safety detectors increases. The current generation of hydrogen detectors is large in size and generally relies on an electrical readout at the area of measurement.
We are developing various hydrogen sensor concepts:
1. Optical fiber based threshold detectors using metal-hydride based sensing layers at the top of an optical fiber. Optical fibers have the advantage that they can be read at a distance and - due to their small size- many of them can be readout in parallel with a single detector. To detect hydrogen we us a thin layer- e.g. a Magnesium-Titanium alloy- deposited at the end of an optical fiber. At a well-defined hydrogen pressure this switchable mirror transforms into a highly absorbing state (picture at the left). This defines the threshold pressure of the sensor. The change in reflection is measured by shining red diode from the other end of the fiber and measure its reflection by a photo-detector. The detection speed is <20 s and the switching lifetime at least 200 cycles.

For the catalytic absorbtion of hydrogen a Pdlayer is added. The Pd itself is protected by a sputtered Teflon layer which prevents the formation of an adsorbed water layer when operating the sensor in air. If gasses such as CO are present, other protection layers are needed to prevent the poisoning of the Pd.
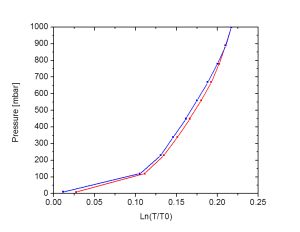
2. To make optical fiber based hydrogen sensors we explore metal hydrides with a continuous change in optical properties with hydrogen concentration. One possibility is to choose materials with a critical temperature below room temperature, such as the Pd85Ta15 alloy shown below.
3. We also explore sensors based on surface plasmon resonance. Below is a schematic representation of the cross-section of an optical fiber on to which a thin gold layer is deposited; the surface plasmon is induced in this gold layer. The resonance frequency depends on the dielectric function of the surrounding material. By changing the SiO2 thickness several resonance frequencies can be generated along a single fiber. The Pd is deposited on top of the SiO2 layer; changes in the dielectric properties of Pd due to hydrogenation has a small but measurable effect on the resonance frequency (dotted lines in right hand figure).
 |  |
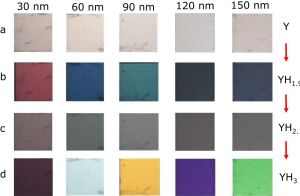
4. Currently, we explore eye-readable hydrogen tape sensors. Here we simply deposit the optical detection layer on a (plastic) piece of tape and cover it with a thick layer of Pd. Upon hydrogenation, color changes are observed due to the interference with the Pd. Taking advantage of the reversible change in optical properties of an Y thin film upon exposure to H2, we detect the presence of hydrogen in concentration range between 5 and 100 ppm H2 in a step-wise fashion. The nature of the color change is a function of the layer thickness.
Funding: This research is funded by NWO/ACTS, STW and third parties.
Applications: Tape sensors for health applications Fiber optic sensors measuring the hydrogen partial pressure in gas mixtures (e.g. natural gas) Fiber optic sensors measuring the hydrogen partial pressure in liquids (e.g. oil)
Links: Martin Slaman’s (the inventor) webpage
Papers:
- Fiber optic hydrogen detectors containing Mg-based metal hydrides
- Optimization of Mg-based fiber optic hydrogen detectors by alloying the catalyst
- Nanostructured Pd-Au based fiber optic sensors for probing hydrogen concentrations in gas mixtures
- A reliable, sensitive and fast optical fiber hydrogen sensor based on surface plasmon resonance
- Seeing Hydrogen in Colors: Low-cost and Highly Sensitive Eye Readable Detectors for Hydrogen Sensing Applications
Collaboration:
- Christoph Langhammer (Chalmers, Sweden)
- Nicolas Javahiraly (University Strasbourg)
Contact:
Bernard Dam
For the development of sensor we use the technique called ‘hydrogenography’
The concept:
Hydrogenography is a combinatorial thin film technique which allows us to screen the thermodynamic and kinetic properties of hydrogen chemisorption in metal hydrides optically. After deposition of a compositional gradient thin film we can analyze the hydrogenation properties of each metal alloy composition in this film simultaneously. The trick is to use the change in optical transmission on hydrogenation. Roughly speaking, this change is proportional to the amount of hydrogen absorbed by the metal.
Placing the thin film in a cell in which the temperature and hydrogen pressure are controlled, we are able to analyze the compositional dependence of the hydrogenation pressure over a wide compositional range.
Phase transformations are easily detected by a sudden change in transparency. From the dependence of the equilibrium pressure on the temperature, we obtain the enthalpy change associated with this phase transformation

On the left: The equilibrium pressure of the phase transformation on hydrogenation as a function of the composition in the Mg-Ti-Ni system at e.g. 400K. On the right: The enthalpy of this phase transformation obtained from the temperature dependence of the equilibrium pressures on the basis of a Van ‘t Hoff analysis
Presently we use this method to:
- Investigate various ways to tune the vapor pressure of the hydrogenation process in order to find materials which can be used to store hydrogen for automotive applications; Investigate the kinetics of hydrogenation in relation to the catalysts used.
- Investigate the properties of metal hydrides in view of their application as optical fiber sensing materials, hydrogen separation membranes, hydrogen storage materials, photochromic materials
Key Papers:
- Hydrogenography: an optical combinatorial method to find new light-weight hydrogen storage materials
- Destabilization of the Mg-H System through Elastic Constraints
- Interface Energy Controlled Thermodynamics of Nanoscale Metal Hydrides
- Combinatorial method for direct measurements of the intrinsic hydrogen permeability of separation membrane materials
- Seeing Hydrogen in Colors: Low-cost and Highly Sensitive Eye Readable Detectors for Hydrogen Sensing Applications
Contact: Bernard Dam