Activate high contrast
To main content
News and Events
Limits of Defect Tolerance in Perovskite Nanochrystals
Lead halide perovskites are a peculiar class of semiconductors. Perovskite solar cells have skyrocketed to efficiencies of 26% in a few years. Perovskite nanocrystals can have photoluminescence quantum yields of 100%, making them highly promising for applications in e.g. lighting and lasing. All this is usually explained by the “defect tolerance” of the perovskite electronic structure: as a result of the specific atomic orbitals that join to form the conduction and valence bands, structural defects or undercoordinated surface ions should not lead to electronic states in the bandgap, so-called “traps”, which usually lead to all sorts of trouble. And yet, both theoretical and experimental studies show that undercoordinated halide ions on the surface do sometimes form traps. Indy du Fossé and coworkers from the NCFun group of prof. Arjan Houtepen have shown that the traditional picture of defect tolerance is incomplete and should also include the local electrostatic environment. In most semiconductor materials, undercoordinated atoms lead to localized electronic states that lie somewhere in the bandgap. These trap states cause non-radiative recombination of electrons and holes and thus lower the efficiency of a device you build with these materials. Lead halide perovskites, like CsPbBr 3 , however, behave differently because of their defect tolerance. Due to the electronic structure of the valence and conduction band, the traps from undercoordinated atoms are expected to lie outside the bandgap, making them a whole lot less troublesome. However, various studies have shown that undercoordinated bromide atoms still lead to traps inside the bandgap, which cannot be explained by the traditional picture of defect tolerance. In their recent publication in the Journal of the American Chemical Society , Du Fossé and colleagues explain this discrepancy by showing that you should also take the local electrostatic potential into account. An undercoordinated bromide at the surface of the nanocrystal can experience a different potential than a bromide somewhere in the middle of the particle. This potential can push the traps related to the surface bromide from inside the valence band all the way up into the bandgap. Not only do these findings increase our understanding of traps in lead halide perovskites, they also point the way to new methods for removing these unwanted traps. Usually, one tries to covalently bind another molecule (a “ligand”) to an undercoordinated bromide to remove the trap state. Du Fossé and coworkers show that simply adding a positive ion near the bromide also does the trick. It lowers the electrostatic potential around the bromide and thus pushes the trap out of the bandgap. Limits of Defect Tolerance in Perovskite Nanocrystals: Effect of Local Electrostatic Potential on Trap States – Du Fossé et al., J. Am. Chem. Soc. Indy du Fossé Prof. Arjan Houtepen Read the article
Jan-Maarten Geertman Alumnus of the Year 2022
Alumnus of the Year 2022 Jan-Maarten Geertman, Director of Net Zero Production at Heineken, has been elected TU Delft Alumnus of the Year 2022. Rector Magnificus and Chairman of the Executive Board Tim van der Hagen will present Geertman with the award during the TU Delft for Life | Xperience Day on 9 June. The event will take place live on campus for the first time since 2019. TU Delft Alumnus van het Jaar 2022 Jan-Maarten Geertman is the embodiment of what we call 'TU Delft for Life'. As a sustainable engineer, he brings (Delft) knowledge to society. As a committed alumnus he has never lost touch with our university but puts his experience at the service of new generations of engineers. ― Prof. dr. Tim van der Hagen, Rector Magnificus en Voorzitter van het College van Bestuur "We hope to maintain these close ties in the coming years. We would also like to continue exchanging expertise in the run-up to a climate-neutral campus in 2030", says Tim van der Hagen, Rector Magnificus and Chairman of the Executive Board . Jan-Maarten Geertman studied Chemical Engineering and obtained his doctorate in 2006 for his research on improving fermentation processes for the food industry. Achieving sustainability by improving processes and value chains has been central to his career ever since. As a lead investigator with the Canadian company Logen, he led research projects into the production of biofuels from renewable agricultural raw materials. Heineken Between 2010 and 2015, Geertman led the yeast research programme at Heineken. During that time, he was responsible for establishing national and international public-private research partnerships in which TU Delft was often one of the research partners. He was behind several successful innovations after 2015, including non-alcoholic beer and the introduction of new product lines, whereby increasing the sustainability of beer production through process intensification played an increasingly important role in his work. Between 2018 and 2022 – during the Brexit period – Geertman was Manager of Supply Chain Support at Heineken UK in Manchester. Here, too, he was responsible for process innovation in the field of sustainability. In the ‘Green Grip’ project, for example, plastic beer can packaging was completely replaced with renewable, biodegradable materials at three large production sites. Since this year, he has been leading the transition to climate-neutral beer production at all of Heineken’s 188 production sites worldwide, to be achieved by 2030. Eliminating greenhouse gases plays a role in the entire food industry, so Geertman’s influence as a pioneer extends far beyond the brewing sector. Staying involved with TU Delft Throughout his highly successful career in the industry, he has always remained involved with TU Delft by supporting the university’s research and education. He is currently the chairman of the Biotechnology Academy Delft, which organises post-initial education through the Biotechnology department, including advanced courses for researchers and PhD students from the industry. He also regularly serves as a sounding board for individual researchers, for example on career issues. Alumnus van het Jaar TU Delft and the Delft University Fund established the ‘Alumnus of the Year’ prize in 2011 to recognise alumni who have made their mark in the world of innovation and research. The winners get a plaque on the Alumni Walk of Fame in Mekel Park on the TU Delft campus. Information and contact Angeline Westbroek, Alumni Relations team leader, mobile phone +31 (6) 1401 5032. For more information on TU Delft for Life | Xperience Day on 9 June 2022 and on Alumnus of the Year, check: TU Delft for Life | Xperience Day 9 June 2022 Alumnus of the Year
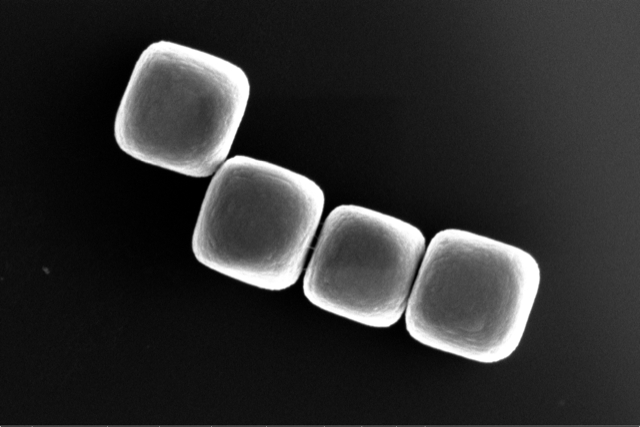
New route to build materials out of tiny particles
Researcher Laura Rossi and her group at TU Delft have found a new way to build synthetic materials out of tiny glass particles – so-called colloids. Together with their colleagues from Queen’s University and the University of Amsterdam, they showed that they can simply use the shape of these colloids to make interesting building blocks for new materials, regardless of other properties of the colloidal particles. Rossi: “This is striking, because it opens up a completely new way to think about materials design.” Their work is published in Science Advances today. Colloids are tiny particles, a few nanometres to a few microns in size. They consist of a collection of molecules and can have different properties depending on the material they are made of. “Under certain circumstances colloids can behave like atoms and molecules, but their interactions are less strong,” Rossi explains. “That makes them promising building blocks for new materials, for example for interactive materials that can adapt their properties to their environment.” New way of materials design If left alone, the cube-shaped colloids from this study, which are made from glass, will assemble themselves into simple structures like distorted cubic and hexagonal lattices. But instead of going immediately from the building block to the final structure, the researchers took small groups of colloids and combined them into bigger building blocks. When they assembled these clusters of colloids, they ended up with a different final structure with different material properties than the self-assembled structure. “From a chemistry point of view, we always focus on how we can produce a certain type of colloid,” Rossi says. “In this study, we’ve shifted our focus to: how can we use the colloids that are already available to make interesting building blocks?” A step forward According to Rossi and her collaborator Greg van Anders, one of the ultimate goals of their research community is to design complex colloidal structures on demand. Rossi: “What we found here is very important, because for possible applications, we need to have procedures that can be scaled up, which is something that will be hard to achieve with most currently available approaches.” “The basic ability to pre-assemble identical pieces from different building blocks, and have them make the same structure, or to take the same building block and pre-assemble different pieces that make different structures, are really the basic ‘chess moves’ for engineering complex structures,” adds Van Anders. Although Rossi studies the fundamental aspects rather than the applications of materials design, she can envision eventual applications for this specific work: “We found that the density of the structure that we prepared was much lower than the density of the structure you would obtain by using the starting building blocks. So you can think about strong but lightweight materials for transportation.” Teaming up After Rossi’s team built clusters of colloids in the lab, they relied on the team of Greg van Anders from Queen’s University to build the final structure out of pre-assembled clusters with a computer simulation. “With these kinds of projects, it’s great to be able to team up with others who can run simulations, not only to understand what’s happening in depth, but also to test how big the chance of a successful lab experiment will be,” Rossi explains. “And in this case, we got very convincing results that we were understanding the design process well and that the resulting material can be useful.” The next step will be to actually build the final structure made from the groups of colloids in the lab. “After seeing these results, I’m confident that it can be done,” says Rossi. “It would be great to have a physical version of this material and hold it in my hand.” Four cubic colloids made from glass Assistant Professor Laura Rossi L.Rossi@tudelft.nl Rossi group Assistant Professor Greg van Anders gva@queensu.ca Greg van Anders' Group
This content is being blocked for you because it contains cookies. Would you like to view this content? By clicking here , you will automatically allow the use of cookies.