Fission products chemistry in SFRs
The safety of a nuclear reactor is directly linked to the performance of the nuclear fuel under normal operating and accidental conditions. This nuclear fuel is subjected to extreme conditions of temperatures and gradients, a corrosive environment, and damage from high energy particles from fission. In addition, fission products such as xenon (Xe), krypton (Kr), cesium (Cs), iodine (I), tellurium (Te), molybdenum (Mo), barium (Ba), strontium (Sr) etc, are generated during irradiation, which makes the chemistry of the mixed oxide (MOX) nuclear fuel particularly complex. Some of the fission products are soluble into the fuel matrix, while others form gas, metallic or oxide precipitates depending on conditions of temperature and oxygen potential in the reactor.
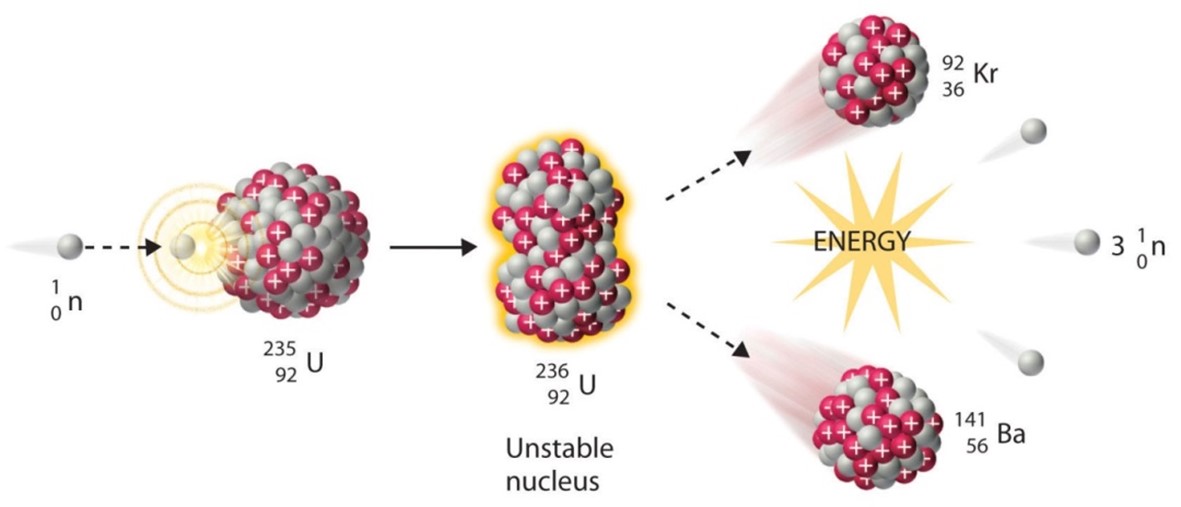
Cs, Mo, I and Te in particular are volatile fission products that are generated with a high fission yield. They migrate in a fast neutron reactor (e.g. SFR or LFR) from the centre of the fuel pin towards the pellet edge due to their high volatility, and to the strong temperature gradient (~450 K mm-1). They accumulate in the space between fuel and cladding, forming a multi-component phase, the so called “Joint-Oxide Gain” (JOG), in the form of a layer of 150-200 μm. Cesium molybdate Cs2MoO4 is the main component of the JOG, which shows a high and anisotropic thermal expansion, which is detrimental to the mechanical properties, and a thermal conductivity one order of magnitude lower than the fuel, which can lead to the creation of hot spots.
The chemistry of cesium compounds forming in the JOG is particularly relevant since the potential release of 135Cs and 137 Cs (the isotopes which are the main cause for the radiological consequences of an accident) into the environment is a subject of primary concern for the public. At TU Delft we investigate in the radioactive laboratories of the Reactor Institute Delft the complex chemistry of the JOG combining structure studies by X-ray and neutron diffraction, X-ray Absorption Spectroscopy (XAS), thermodynamic measurements by Differential Scanning Calorimetry (DSC) and solution calorimetry, as well as thermodynamic modelling assessments using the CALPHAD method.
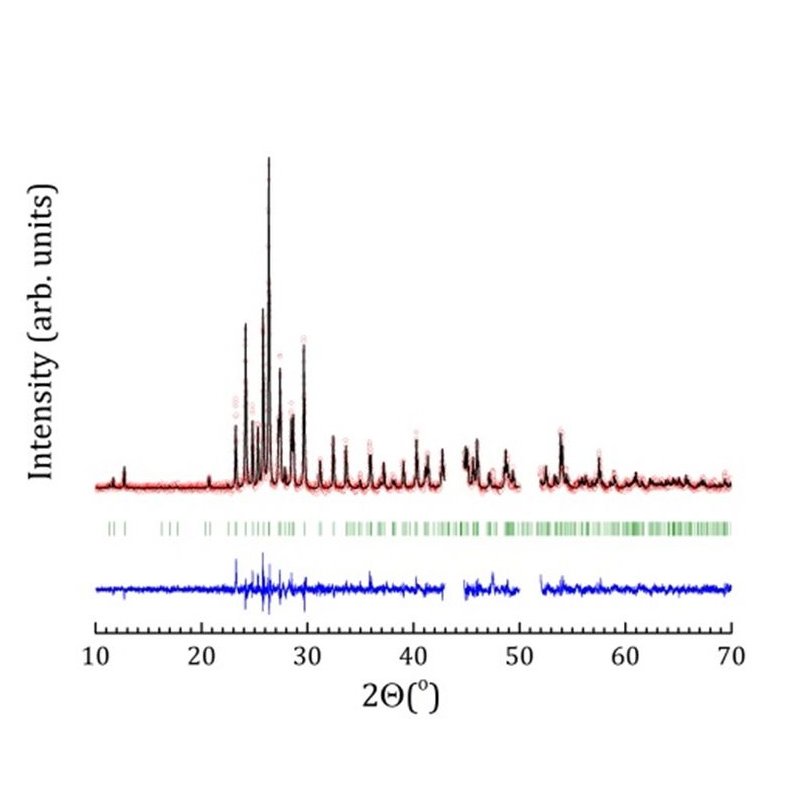
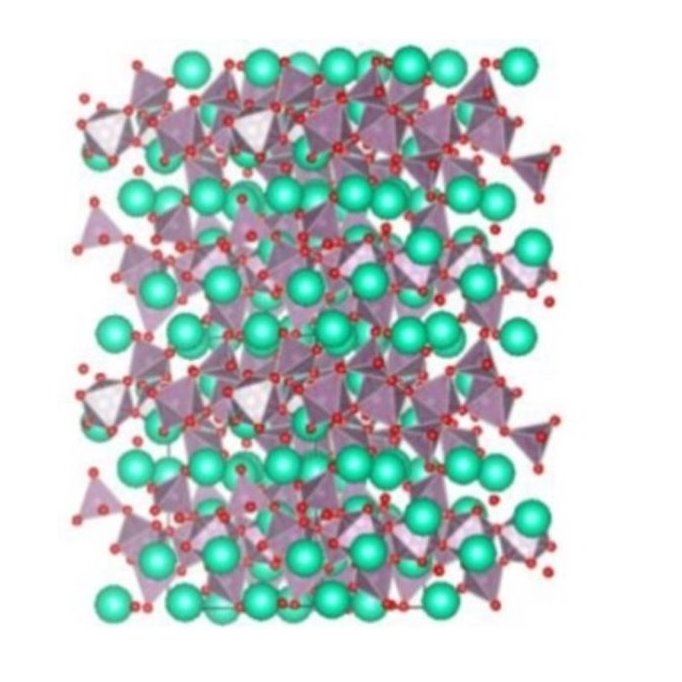
X-ray diffraction patterns of β-Cs2Mo2O7 at T=683 K (b) Sketch of the structure of α-Cs2Mo2O7 at room temperature
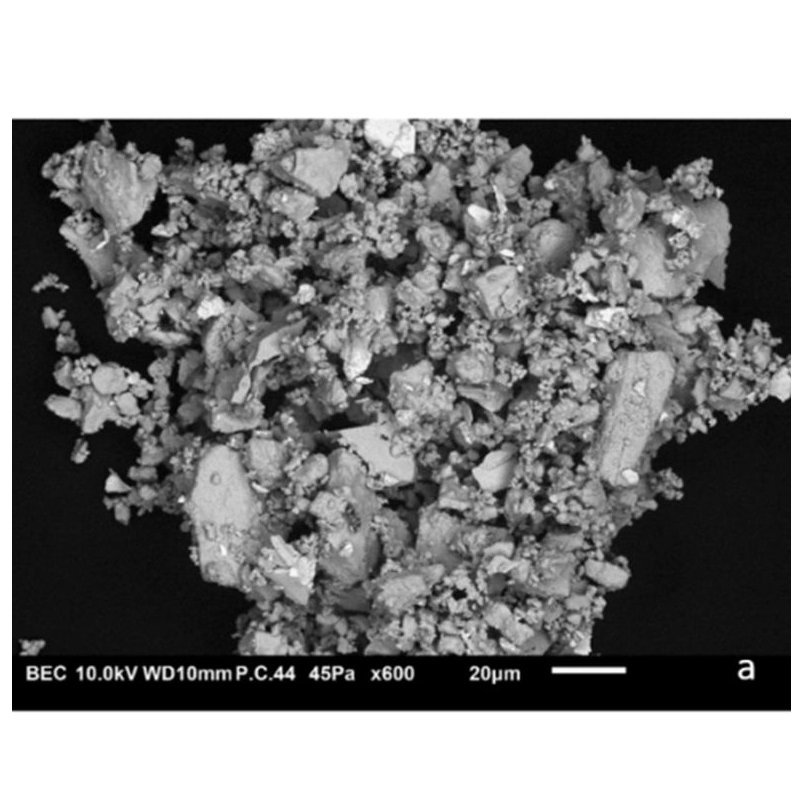
(c) SEM picture of α-Cs2Mo2O7
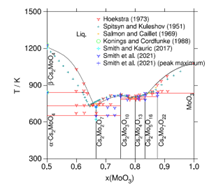
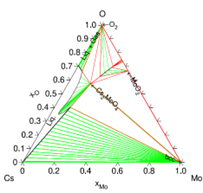
Thermodynamic model of the Cs-Mo-O system
References
E. Epifano, A. Volfi, M. Abbink, H. Nieuwland, L. van Eijck, G. Wallez, D. Banerjee, P.M. Martin, Anna L. Smith, Investigation of the Cs2(Mo,Te)O4 Solid Solution and Implications on the Joint Oxyde-Gaine System in Fast Neutron Reactors, Inorganic Chemistry, 59(14) (2020) 10172–10184, doi.org/10.1021/acs.inorgchem.0c01307
A.L. Smith, T.N. Pham Thi, C. Gueneau, J.-C. Dumas, E. Epifano, W. van Burik, N. Dupin, Thermodynamic modelling assessment of the ternary system CS-Mo-O, Calphad 75 (2021) 102350, doi.org/10.1016/j.calphad.2021.102350