Electrochemical conversion (transforming chemicals using electricity) is a promising sustainable technology for converting waste CO2 into useful materials and fuels. Isabell Bagemihl developed a multi-scale model – from reaction channel to process-scale – to help guide fundamental electrolyser research, bringing a closed carbon cycle ever closer.
Operating at ambient temperature and pressure and solely with the input of renewable electricity, electrochemical conversion processes can operate with a lower environmental impact compared to combustion processes. And with electrochemical CO2 conversion proven feasible on a laboratory scale, research is now concentrating on refining design and operation of industrial scale devices (called electrolysers).
A challenging step forward, as scaling up electrochemical CO2 conversion comes with both technical and economic uncertainties. Fascinated by applying scientific principles to real-world challenges, particularly in areas like energy and environmental sustainability, Isabell Bagemihl was eager to help make a difference.
Scaling up electrochemical CO2 conversion comes with both technical and economic uncertainties.
Similar, but not
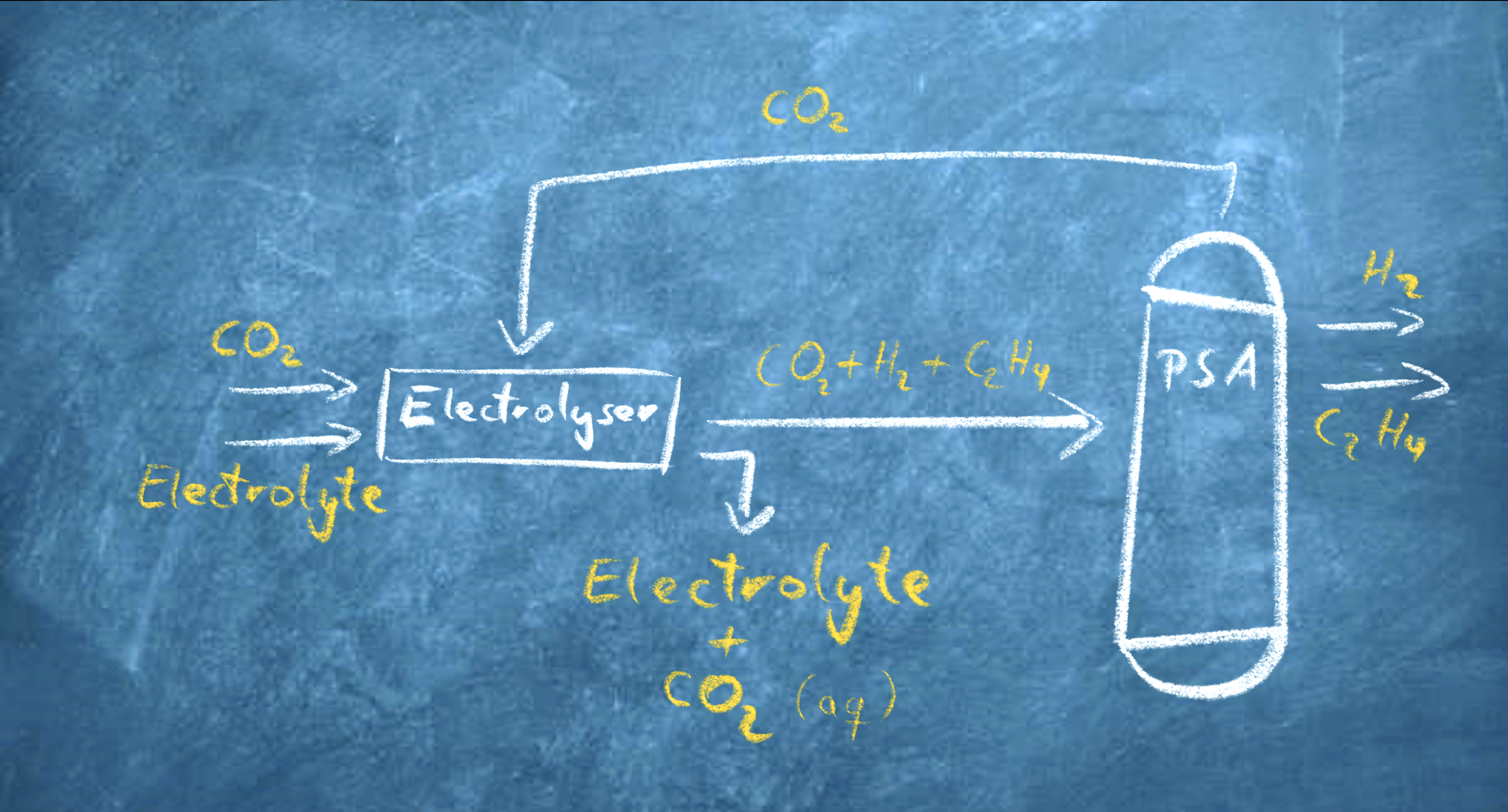
In an electrolyser, CO2 must reach a catalyst where it will be converted into another molecule. This requires energy, in the form of electricity. The reaction product must then move away from the catalyst to make place for more CO2 to be converted. ‘Even though using similar lab-scale setups, existing studies have reported quite different values for performance measures related to the efficiency of this process,’ says Bagemihl, PhD-candidate in the Product and Process Engineering group at the Applied Sciences Faculty. ‘We wanted to increase our understanding of maximum operational efficiency for existing electrolyser designs for CO2 conversion and bridge the gap between reactor design and economic viability. Until now, these two worlds existed independent of each other.’
Crossing the channel
The main challenge her research addresses is that there are interdependencies between various performance variables, such as how fast and efficient the reaction runs, and if the electrochemical process only yields the desired end-product or also other products. ‘These interdependencies will propagate – and may change – from the lab-scale to the scale of an industrial process, thereby influencing economic viability,’ Bagemihl says. ‘My master student Lucas Cammann did a wonderful job at developing model that captures the underlying mass-transfer effects – the “coming and going” of reactants and reaction products – at multiple scales.’
The smallest scale of the model is that of an individual electrolysis channel through which both reactants and reaction products are transported. At this scale, the model simulates gas movement, substances spreading, and their concentrations changing over the length of the channel. This subsequently allows prediction of performance at the scale of an electrolyser, which contains thousands of such channels. The largest scale is the process-scale, which includes a CO2 feed source, the electrolyser, and a subsequent gas separation unit. All these steps are required for the successful implementation of electrochemical conversion in a practical factory setting.
Economic trade-off
While process-scale models for economic assessment of electrochemical conversion processes did already exist, they typically assume the electrolyser to be a black box of which the various performance variables can be tuned independently of each other. Bagemihl: ‘By explicitly integrating the electrolyser-scale and channel-scale in our framework, we can analyse the economic trade-off that comes with the interdependency between the various reactor performance variables. You can, for example, have a very high reaction rate which helps save on investment costs. Or you can have a very high specificity towards the desired product, thereby reducing downstream separation costs of the product. But you can’t have both at the same time.’
Demonstrating the model for a single reaction (CO2 -> ethylene) for example showed that economically optimal current densities at the process-scale – and thereby the reaction rate – can be as low as half of the previously reported benchmarks. ‘It shows that the economics don’t yet match. This emphasises the importance of using multi-scale models to better understand the obstacles in creating economically viable system designs.’
The economics of electrochemical conversion of CO2 don’t yet match.
Plug and play
A big strength of her research, and one that helped her win the Best Energy Paper award, is that the model is generic. It can be applied to various electrolyser designs and processes yielding different end-products. Bagemihl: ‘The same framework can be applied, whether it is an electrochemical conversion process yielding carbon monoxide, formate or ethanol. One only needs to plug in what is happening inside the reactor, so others can quickly benefit.’
This is where we’re at
The model being based on simplified reaction mechanisms and design considerations, Bagemihl stresses that the optimal performance values she reports should not be interpreted as “this is what everybody should now aim to achieve”. ‘Our study merely shows where we are at with current reactor designs. With the insights it provides, we can now tweak electrochemical conversion from both the reactor side and the economics side.’
Bagemihl found it both a challenge and inspirational to do research on new technology that can help address the pressing global issue of climate change. She hopes the model will help guide research performed at the lab scale; that people will no longer optimise on one single performance value only but rather assess and report all operating conditions. Providing researchers and potential investors the means to identify and address bottlenecks early on can only speed up the green transition, bringing a closed carbon cycle ever closer.
Our model provides researchers and potential investors the means to identify and address bottlenecks early on.
Read Isabell's winning publication in its entirety here.
The Best Climate and Energy Paper Award 2023 Ceremony took place on Tuesday 19 March 2024. Curious about the other eight finalists? See the full press release sent out at the time here.
If you would like to interview one of the finalists, or learn more about Delft's climate and energy ambitions, please feel free to contact:
• Dave Boomkens, science information officer Climate & Energy - 06 3408 1461 / d.j.boomkens@tudelft.nl
• Floris den Broeder, science information Climate & Energy - 06 4520 7074 / f.denbroeder@tudelft.nl