A brief history of Quantum
Although quantum technology is relatively new, its history stretches back further than you might think.
The Origins of Quantum
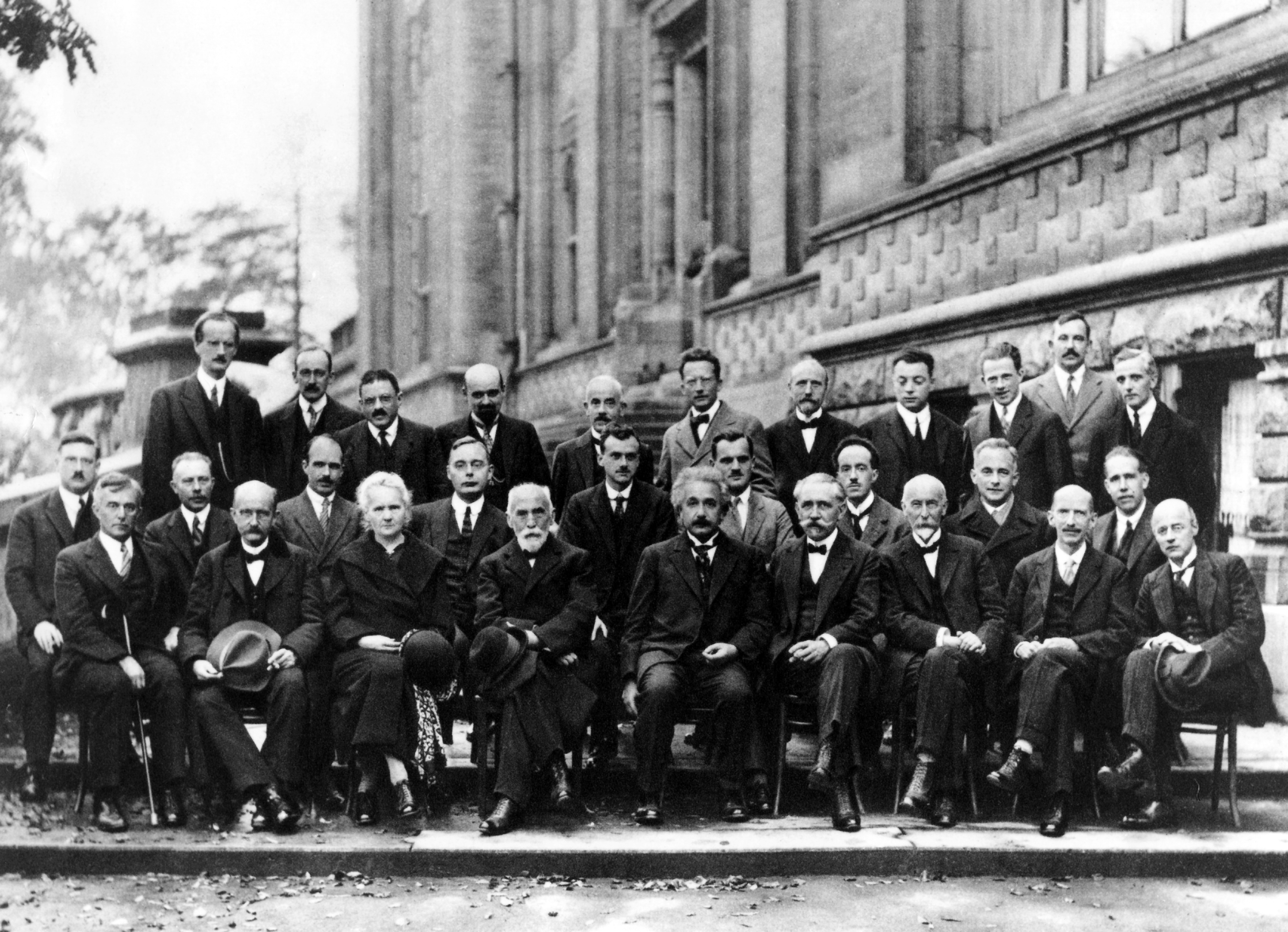
What makes something “quantum”? The first scientist to answer this question was the 23 year old Werner Heisenberg, a student of Niels Bohr. Prior to 1925, Bohr had put forward a mix of old and new ideas in physics that seemed to explain extremely well how the hydrogen atom worked. But there was much confusion as to why the model worked so well. It was Heisenberg who first connected the dots. His inspiration was to focus only on what can be observed.
This subtle idea separates the quantum world – one of small things and small energies – from the larger objects of our normal, or classical, world, which includes grains of sand, coffee cups and planets. For example, consider the moon. On a clear night, we can see light from the sun brightly reflected off of it. On overcast nights, or when it is below the horizon, we can’t see it at all, but we know it’s somewhere. Astronomers who carefully collect data can predict with extremely high accuracy exactly where in the night sky it will appear a great deal into the future. Very small things, such as the electrons in atoms and molecules, do not behave this way. They are not always somewhere. If you assume that an electron is in one particular place when you aren’t immediately observing it, you will not be able to explain a great deal of natural phenomena.
The quantum idea is a radical departure from our usual intuition: it predicts concepts like superposition and entanglement that we do not experience in everyday life. In spite of that, quantum mechanics has proven to be the most accurate physical theory ever conceived, and now underpins our knowledge of far more than the humble hydrogen atom. For instance, it is essential for understanding the operation of the semiconductor chips inside computers, and how chemicals, such as our DNA, bond together. In less than a century, quantum mechanics has transformed from a strange idea into a cornerstone of modern technology.
1925
Understanding Hydrogen
1938
Nuclear fission was discovered in 1938. The process of the nucleus – the quantum-mechanical innards of an atom – splitting apart would go on to be harnessed for power generation (as well as nuclear warheads).
1947
Transistors – the essential building blocks of modern computers – are the most numerous objects created by humanity. Quantum mechanics is necessary to design these small and ubiquitous devices, found in laptops, smartphones and cars.
1954
Solar cells work by converting light energy into electrical energy. Understanding this quantum process is critical to building these efficient renewable energy sources.
1960
Lasers are near-perfect sources of light that are engineered into everyday devices, such as printers and barcode scanners. They are an essential tool in much of modern science, including approaches to building quantum computers.
1971
MRI - What is now a commonplace medical scan is really quite high-tech. Magnetic fields and radio waves are used to accurately image tissue by proving the behavior of proton spins: little quantum systems in the body. What’s more, the strong magnets required are typically superconducting – another quantum behavior.
1990s
Flash Memory - Many USB sticks make use of flash memory: memory that can easily be erased with a single ‘flash’ of voltage. Flash memory makes use of the effect that quantum particles can pass seemingly insurmountable barriers – a phenomenon known as quantum tunneling.