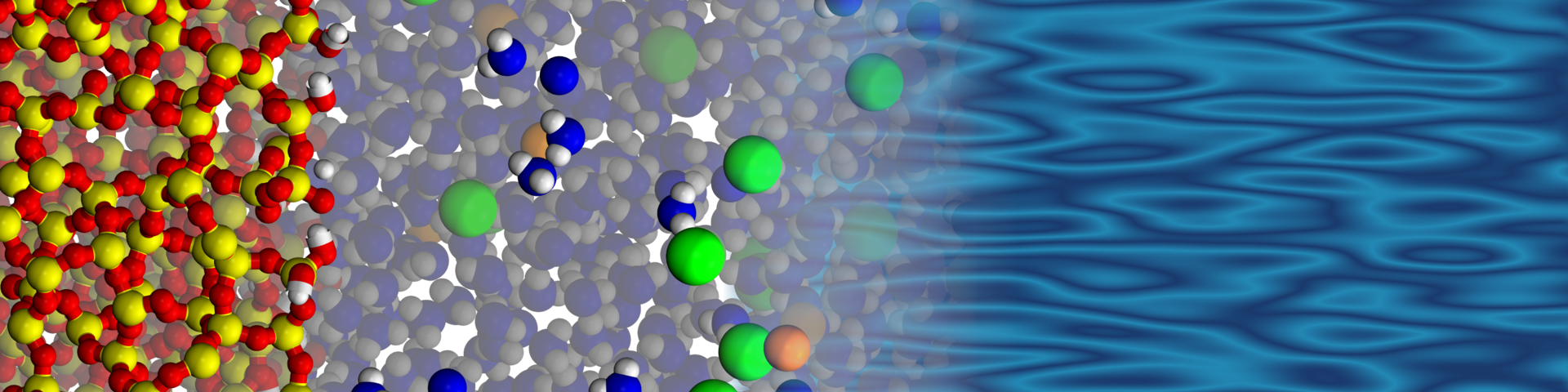
Simulations to make insight into electrokinetic transport more reliable
Researcher Remco Hartkamp and PhD student Max Döpke of the Process & Energy Department have taken an important step in making simulation results for electrokinetic transport more reliable by using molecular simulations. In electrokinetics, ions play an important role in the transport of a liquid through narrow pores or of solid particles through a liquid. These types of transport properties are important in many nanotechnological or electrochemical applications and in colloidal suspensions for example for pharmaceutical applications. The research results were published this week in The Journal of Chemical Physics.
Electrokinetic transport is being actively investigated both experimentally and with the aid of molecular dynamics simulations, sometimes with highly divergent results. These divergent results make it difficult to determine what is correct. Experimental measurements are often interpreted by using models that can be highly accurate under certain conditions, but highly inaccurate under other conditions. Hartkamp and Döpke use molecular simulations in which they have access to more detailed data at the molecular level. The interpretation of such simulations does not rely on models, making simulations an important tool to better understand electrokinetic properties and to enable better interpretations of experiments. However, molecular simulations rely on a correct description of molecular interactions. The researchers discovered that electrokinetic properties depend strongly on the molecular interactions between solids and ions. They subsequently adapted these interactions in such a way that the simulation results corresponded closely to experimental data under unique conditions where there is little doubt about the interpretation.
Remco Hartkamp: ‘Thanks to this new approach, we can be confident that our simulations reflect reality. This, in turn, can be used to gain insight under conditions where experimental studies do not provide a clear and consistent picture. In addition, it allows us to “see” exactly how ions and water molecules are distributed and how they move near a solid surface.’

R.M. (Remco) Hartkamp