Michel Eppink Group
Bibliography
Michel Eppink has more than 35 years of experience in Downstream Processing of large biomolecules (proteins, lipids, carbohydrates).
Michel received his MSc. in Biology/Chemistry in 1993 from the University of Utrecht and his PhD in Biochemistry in 1999 from the Wageningen University and Research at the Laboratory of Biochemistry with background in structure/function relationships of redox proteins.
After that Michel moved to the industry at Organon NV thereby starting as a trainee followed by assistant Section Manager and finally Section Manager Downstream Processing Research leading a team of 20 highly skilled coworkers developing purification processes for biopharmaceutical proteins such as hormones for diseases like diabetes and fertility. In 2009 Michel moved to Byondis BV (formerly known as Synthon BV and Synthon Biopharmaceuticals BV) becoming Director and finally Senior Director Downstream Processing heading a department of 30 highly skilled coworkers developing purification / formulation processes for biopharmaceutical proteins such as monoclonal antibodies and antibody drug conjugates for diseases as oncology.
From 2014 - 2024 he was part-time full Professor at the Department of Bioprocess Engineering at Wageningen University and Research heading a group of PhD candidates in the research of biorefinery technologies such as new purification / analytical techniques for proteins / lipids / carbohydrates / pigments from different eukaryotic organisms (e.g. mammalian cell lines, macroalgae, microalgae, yeast).
In 2024 Michel started as Special Professor at the Department of Biotechnology of TU Delft with focus on “Industrial Biopharmaceutical Downstream Processing”.
Definition
“Industrial Biopharmaceutical Downstream Processing” is a technology driven position that integrates harvesting, cell disruption, extraction, conversion, separation and formulation technologies of biopharmaceuticals within Bioprocess Engineering. The downstream processing needs to be mild and efficient at the same time in order to maintain the functionality of the products (e.g. native protein conformation) and thus value. Biopharmaceuticals are ingredients for a variety of medical applications (e.g. oncology, inflammatory, autoimmune diseases) and to cope with the worldwide high prices of medicines efficient and lean biopharmaceutical processes should be developed in the coming decades to lower the cost of goods of these medicines.
Aim
The aim of the new professor (Part-time) in the coming years is to build more expertise in the downstream part of biopharmaceutical processes aligning with the biopharmaceutical work that is already established in the Bioprocess Engineering section (BPE) of the Department of Biotechnology, giving these activities even more critical mass and making BPE the “to-go-to” one-stop-shop for Biopharmaceuticals Process Development. It ties in well with the pharma focus of the Biotechnology Department, and the pharma initiative of TU Delft’s Bioengineering Institute. Moreover, green alternatives will be investigated dealing with the biobased economy and circularity.
My dream would be to understand at molecular level and use this understanding to develop lean production processes for biopharmaceuticals (e.g. monoclonal antibodies, antibody drug conjugates, other recombinant proteins) from product expression up to final product with a green focus in mind.
Research
DSP is a bottleneck in Process Development, scattered knowledge is available for certain organisms (e.g. bacteria, yeast, fungi, mammalian cells) but not well structured. The focus will be to improve overall process efficiency, selectivity, performance or to be able to integrally and holistically design a pharmaceutical production process. Focusing on both USP and DSP would strengthen and expand the existing expertise within BPE. Figure 1 shows an overview of the different DSP stages (green color indicates a mindset on circularity and green processes).
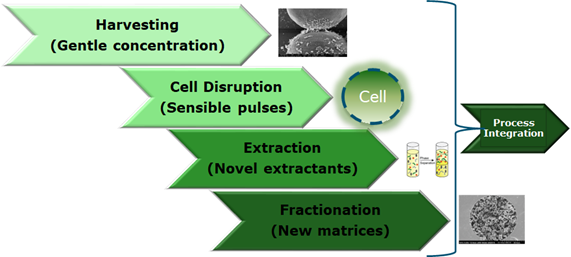
Main topics to be addressed will be centered around fractionation technologies and process integration in newly to be developed processes for biopharmaceuticals.
In the past years I studied different biopharmaceutical processes looking for solutions of specific process steps or the process as a whole that have an influence on product yield/quality, and doing so with a green focus. A selected set of problems were identified for the process integration stage (cTRAC, Continuous Processing, Flow Chemistry) and fractionation stage (Nanofibers, Filtration, Formulation) in the development of biopharmaceutical products as shown below in scheme I from which I think are important bottlenecks on product yield, product quality (Critical Quality Attributes), cost of goods and circularity (green focus). Further investigations are needed to improve process robustness, process efficiency, product improvement such as yield and quality, process understanding, implementation of circularity and waste reduction and decrease overall process time in future biopharmaceutical processes. These studies clearly fit with the expertise present at the TU Delft and strengthen the focus on biopharmaceutical processes.
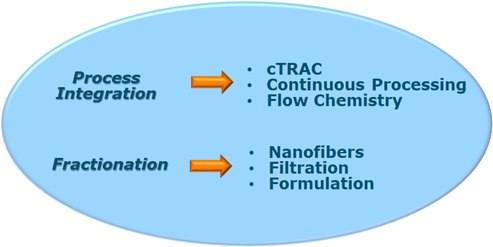
The research topics, as shown in Scheme I, will be investigated for biopharmaceutical proteins (e.g. monoclonal antibodies, antibody drug conjugates).
Education
With respect to education experience has been gained in the past 30 years giving a diverse range of lectures in the field of biotechnology (upstream- and downstream processing, biorefinery of marine organisms) and online lecturing. Currently a few lectures are given:
- Lecturing at advanced course Bioprocess Design led by Prof. Henk Noorman (since 2014) and the advanced course Downstream Processing led Prof. Marcel Ottens (since 2007). These post-initial courses are both organized by the foundation Biotechnology Academy Delft.
- Giving downstream processing lectures at WUR in Wageningen for biopharmaceutical students, Leiden University and Utrecht University for pharmaceutical students and for bioprocess engineering students at Kalsruhe Institute of Technology.
- Providing specific lectures, e.g. Process Analytical Technologies at Radboud University in Nijmegen and Maastricht University.
Education is an important task of the professor position at Bioprocess Engineering of TU Delft. New insights on industrial biopharmaceutical downstream processing technologies will be discussed and implemented focused on complex biomolecules (e.g. biopharmaceutical proteins) and in agreement with the 0.2 FTE position.

Prof. Dr. Michel Eppink
- +31 683169622
- 015 -278 2363
- M.H.M.Eppink@tudelft.nl
-
Building 58, room C2.150