ERC Starting grant for CO2 recycling in chemical industry
Converting large concentrations of carbon dioxide (CO2) into products for the chemical industry. That is what Tom Burdyny, recipient of an ERC Starting Grant, wants to achieve. The method that might make this possible is called electrolysis, which creates new chemical bonds by application of electricity.
CO2 in the chemical industry
The chemical industry uses millions of tonnes of fossil fuels to transform oil and gas into common household items like plastics, cosmetics, and resins. The hydrogen in oil and gas provides an energy source for these transformations, while the carbon atoms form building blocks for these common items. In doing so, the industry emits large concentrations of CO2 and other greenhouse gasses, both as a by-product or when these products reach their end of life. Through electrocatalysis, Burdyny is able to let CO2 react with water, to make building blocks of similar products. This process offers a sustainable alternative to the use of fossil fuels in the large-scale chemical industry.
The field of CO2 reduction with electricity has advanced immensely in recent years. With this grant I want to propose ideas that can lead to stark changes to the status quo, and hoping to inspire others to do the same.
Energy transition
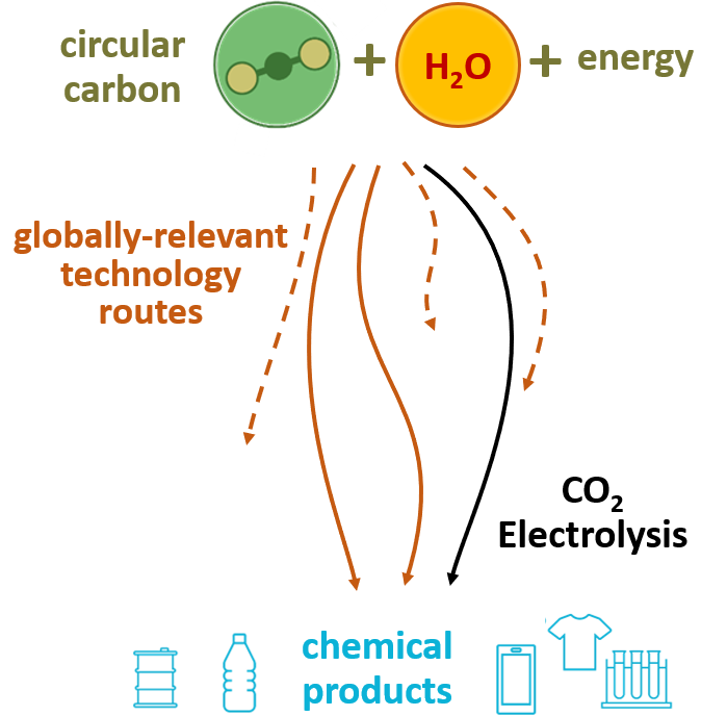
“There are only so many technologies that can convert CO2 into useful fuels and chemicals at globally-relevant scales”, says Burdyny. “These include biomass conversion, water electrolysis combined with traditional chemical engineering, and CO2 electrolysis. While the first two are being implemented at larger scales, we have only recently began to explore the potential for CO2 electrolysis outside of universities. Continued research into this technology is then essential to determine if it will be useful for society in the upcoming energy transition.”
What is unique about the method of electrolysis in Burdyny’s lab, is its simplicity. Only two substances – CO2 and water (H2O) – are needed to make a variety of molecules, and the process does not require any expensive or rare materials, in contrast to modern-day alternatives. Burdyny: “Through this grant our lab will be able to make substantial advancements in both applied and fundamental aspects of CO2 conversion at low temperature, without using any critical raw materials. In particular we’ll be able to create new materials that selectively make different types of high energy content alcohols directly from CO2. It’s easy to transport these alcohols and use them to make other molecules, which offsets the current fossil fuel intensive production routes.”
Black box
While it is clear what goes in and what comes out, the mechanisms behind electrolysis are not clearly understood yet. “Everything we’re doing functions very much like a black box, because we can’t visualise key parts of the system”, Burdyny explains. “A variety of classical control experiments and clever instruments will enable us to look under the hood of these CO2 conversion systems.”
Described as high-risk, high-gain, Burdyny’s research may offer serious applications in a more sustainable chemical industry. His ambition is clear: “Within 5 years, I want to report the first selective production of methanol and ethanol from CO2. These compounds can then function as base molecules for later conversion into products from the multi-billion ton annual carbon-based market.”