Radionuclides are essential in the diagnosis and treatment of cancer and other diseases, because they can detect and destroy specific tumour cells. Yet only a handful of nuclear reactors in the world produce the bulk of these radionuclides. Robin de Kruijff works to solve this medical bottleneck, by developing new ways to produce radionuclides to make sure they become more widely available for medical hospitals and clinics.
In order to improve the quality of targeted tumour therapy, researcher Robin de Kruijff is on a mission to make a larger variety of radionuclides available for clinicians and improve production methods. With her work at the Reactor Institute in Delft (RID) she aims to combat the rigid trend of the last decades, in which the medical use of radionuclides has focused only on a few specific types. The lack of supply has led to the present bottleneck worldwide, which is why a lot of governments and companies are looking for other ways to produce radionuclides.
De Kruijff’s mission is an urgent one: her work may help hospitals become less dependent on the current sparse resources. “Massive improvements in cancer therapy are possible when we start working with other radionuclides than we are currently doing,” De Kruijff starts to explain. “My research focusses on the preclinical development of for example the radionuclides 47Sc, 99Mo, 161Tb and 166Ho, which the clinics can use to design new radiotracers and therapeutics.”
Better quality of care
More variation in medical options will directly improve the quality of treatment for patients, says De Kruijff, who is assistant professor at the RID: “We can then make treatments patient specific. If one type of radionuclide turns out to be more suited to one patient for example, and another type is better suited to another patient, we can test this with a small scout dose and then offer the treatment that works best for that specific patient.”
“Clinicians are just starting to discover the power of variation in radiopharmaceutical treatment for patients and becoming more open to using other diagnostic and therapeutic isotopes,” she continues. “This development made me think: how can we support the clinics in this way? How can we make radionuclides more available and useful for more types of treatment, in order to help these patients in better ways than we currently can? It is very motivating to me that I can see the impact my work might have on all these patient lives.”

Challenges in the medical use of radionuclides
Seventy to eighty percent of all nuclear medicine procedures use the radionuclide technetium-99m (99mTc), which comes from radioactive molybdenum. But the bulk of this molybdenum is produced by only six nuclear reactors in the world – including the HFR in Petten, the Netherlands – and they are regularly shut down for repairs.
The lack of supply is not the only issue halting clinical trials, there are other challenges as well: a high specific activity is required for medical purposes, but radionuclides produced by reactors often show a low specific activity. This means the radionuclide cannot be used in targeted tumour therapy, as receptor sites on the tumour cells will be saturated with non-radioactive isotopes.
The RID at TU Delft features the Higher Education Reactor (Hoger Onderwijs Reactor or HOR), a 2.3 MW pool-type research reactor. At this facility, De Kruijff works on new, smart radionuclide target materials and production pathways which can significantly increase the specific activities of radionuclides to the level required for clinical implementation, with the first applications of 99Mo and 161Tb expected in the 2020s.
Improving radionuclide production
How does radionuclide production work? “When we place different target elements in the reactor – for example iron, copper and gold – they become radioactive,” De Kruijff says. “In the reactor core, neutrons are produced when uranium atoms undergo fission. These neutrons are absorbed by the target atoms, which then become radioactive. By attaching such a radionuclide to a tumour-specific targeting vector, and injecting this so-called radiopharmaceutical into the blood of a cancer patient, the radioactive atom will travel to the tumour site and upon arrival, irradiate and kill the tumour.”

De Kruijff looks at different target materials and separation techniques to improve radionuclide production: “The targets for radionuclide production in nuclear reactors haven’t changed much in the past decades. By rethinking how we do these irradiations, and doing them in a smarter way, I believe we have the potential to really improve both the amount and quality of produced radionuclide. In some cases, for example by using nanomaterials as targets for the aforementioned molybdenum production, we have the added benefit of reducing the amount of co-produced nuclear waste.”
From nuclear research to patient treatment
One of the challenges in De Kruijff’s work, is how to translate her lab-scale research to radioactive materials that can actually be used in hospitals: “In order to apply our research to patient treatments, we need to work with much higher levels or radioactivity than we are currently able to do in our laboratories. Our team works closely together with a number of university medical centers on this, such as Erasmus MC and Radboudumc. We also work with companies: Urenco provides us target material for a molybdenum/technetium generator for example, used to diagnose a lot of diseases. And the American company North Star, one of the biggest companies in radio isotopes, also works with us to discover new ways to produce this molybdenum.”
“For this purpose, we are trying to get subsidies for the installation of what is called ‘hot cells’ in the lab. A hot cell is a workplace with a lot of shielding, which blocks the radioactivity that’s in there. With hot cells we would be able to safely work with much higher levels of radioactivity, which we can make by irradiating our materials for much longer, so we can test whether our small-scale experiments will work for actual patients.”
Back into the blue light
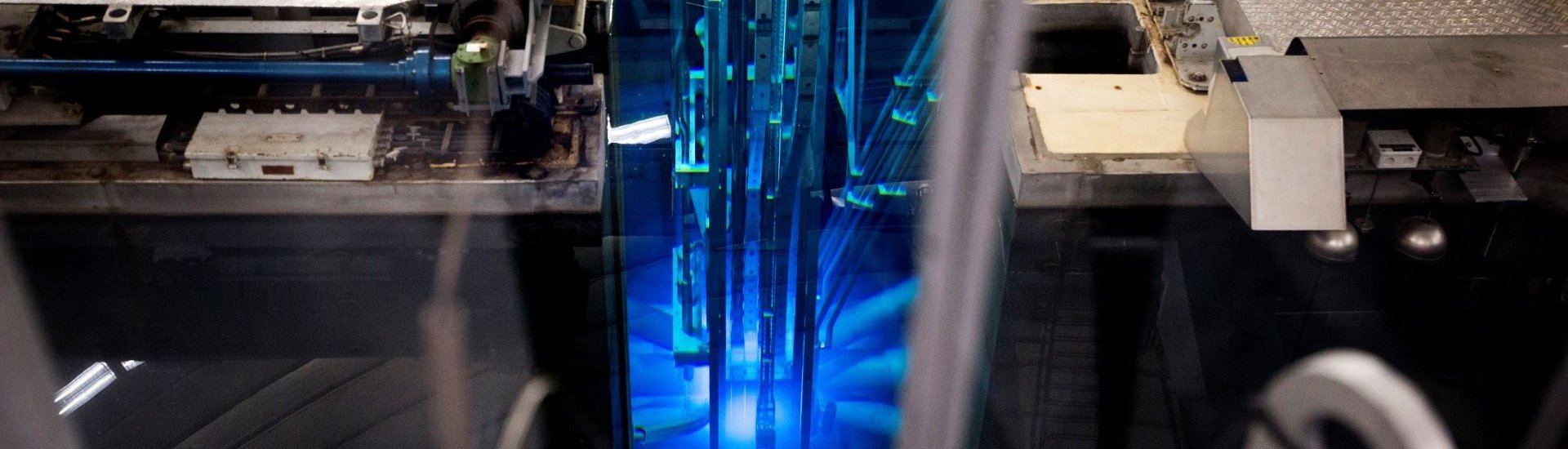
The past two years also brought an unexpected challenge for De Kruijff’s team: the HOR reactor was shut down for upgrades and repairs in 2020 and 2021. De Kruijff grins: “We had to come up with roundabout ways to do our experiments, like ordering radionuclides from companies, or irradiate isotopes at other reactors. However, just before Christmas the first phase of the upgrade was finished, and our reactor was turned on and operating at 2.3 MW again. I had really missed seeing that blue Cherenkov light in the past two years, and am excited to continue my research with our own reactor!”