Brazil and Netherlands Cooperation on the Route to Circular Plastics Economy
The majority of worldwide plastic waste is not recycled. Impediments to the recycling of commodity polymers include poor economics, challenges in separation, impurities and degradation of the macromolecular structures, all of which can negatively affect the properties of recycled materials. An attractive alternative is to transform polymers back into monomers and purify them for repolymerization — a form of chemical recycling we term chemical recycling to monomer. In general, chemicals are employed to promote the depolymerization, however, catalysts and photocatalysts also can be employed to make this process more efficient.
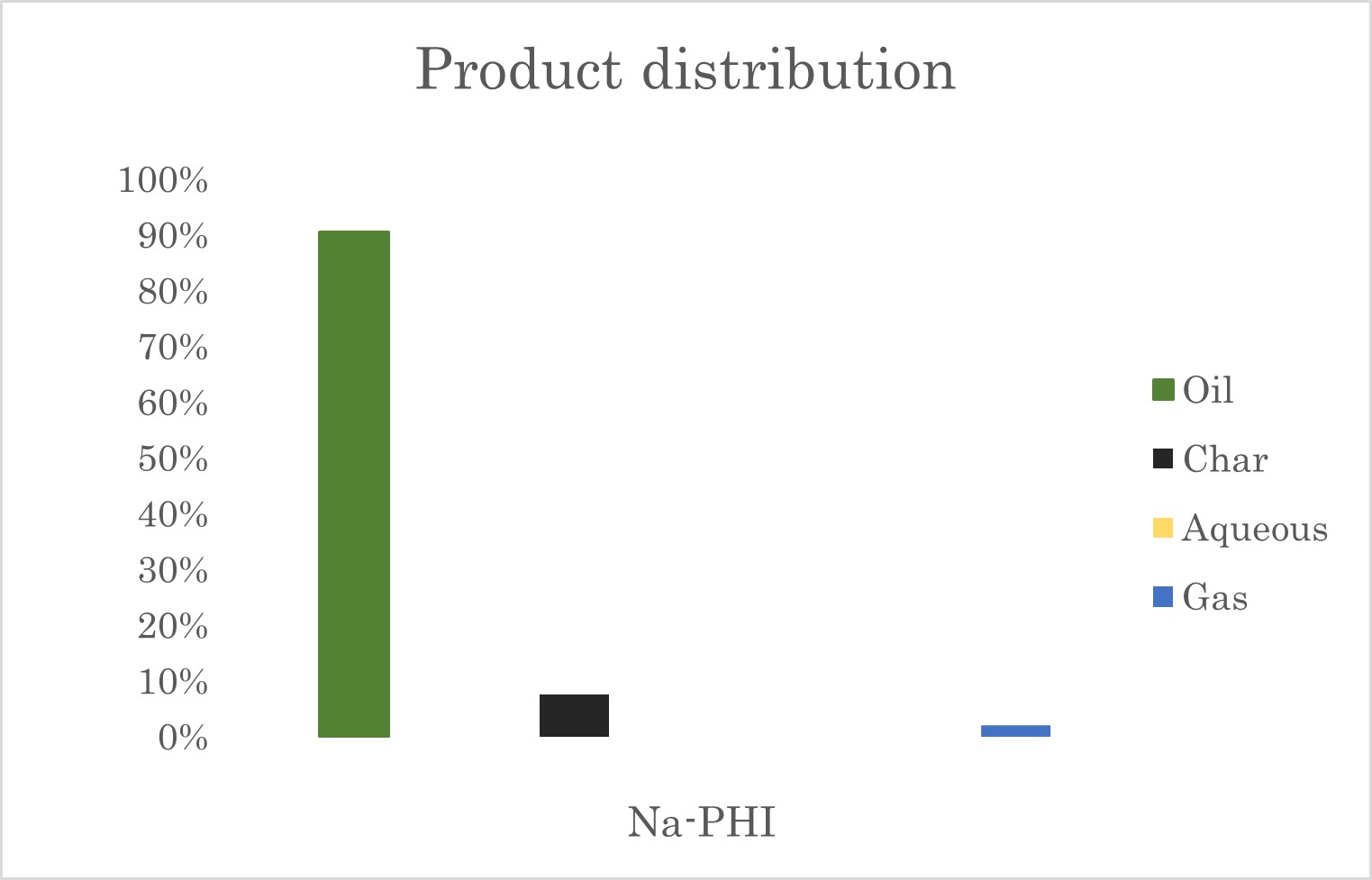
Dr.ir. Luis Cutz (P&E department, Faculty of Mechanical Engineering) and Prof. Dr. Ivo F. Teixeira from Federal University of Sao Carlos (UFSCar) have teamed up to test, for the first time, a novel type of catalyst for waste plastic recycling using hydrothermal liquefaction. Researchers wanted to test a special kind of carbon nitride by reacting an alkali salt, like NaCl, with poly(heptazine imides), also known as Na-PHI. This creates crystal-like structures with highly active sites for a range of reactions.
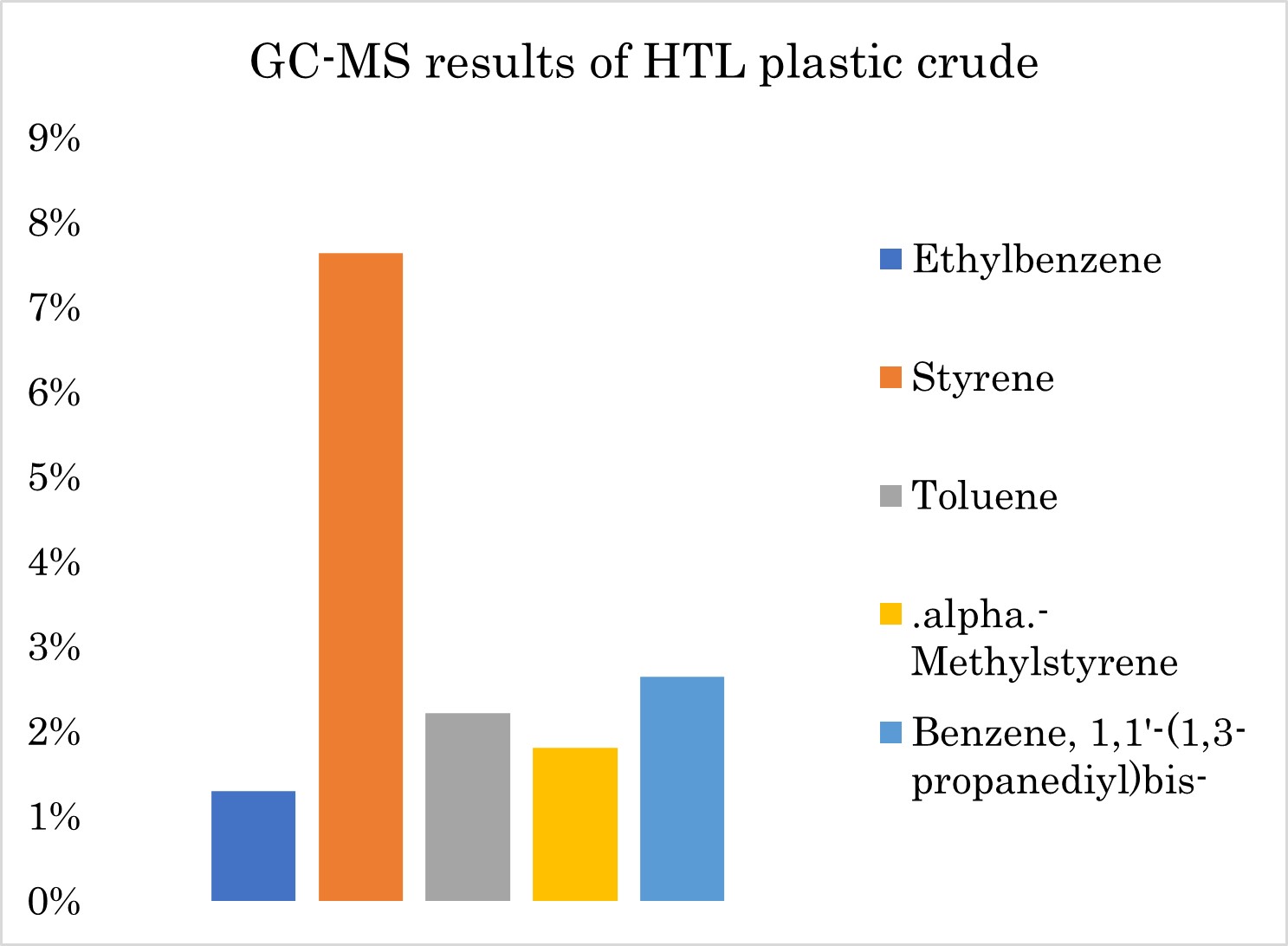
For the chemical recycling tests, polystyrene beads were selected as feedstock, and the chosen process was hydrothermal liquefaction. As can be seen from Figure 1, the plastic crude's high concentration of styrene suggests that Na-PHI successfully breaks down polystyrene and shows Na-PHI’s potential as a catalyst for breaking down polystyrene. However, additional joint research of the process is necessary to enhance the production of styrene or high value chemicals (HVC). Exploring alternative metal-PHI catalysts or different plastics as feedstock may lead to higher monomer recovery rates.
a)
b)
Apart from the tests, Dr. Cutz and TU Delft master’s student Pinelopi Champesi were able to travel to UFSCar in São Paulo to engage in lectures and collaborative work. Also, Prof. Ivo F. Teixeira and his student visited TU Delft in April 2022 where he gave a talk on photocatalysis.
During their time at UFSCar, the TU Delft team visited Prof. Teixeira’s lab to observe the production process of Na-PHI, manufactured by Marcos Ribeiro da Silva (PhD student at UFSCar), in addition to brainstorming potential catalysts for follow-up activities/proposals. Their visit in UFSCar concluded with a seminar at the Chemical Engineering department and a team building dinner at a local restaurant in São Carlos.